Label: LOTEPREDNOL ETABONATE suspension/ drops
- NDC Code(s): 62756-232-55, 62756-232-56, 62756-232-90
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
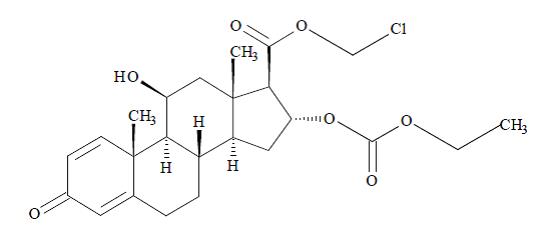
DESCRIPTIONLoteprednol etabonate ophthalmic suspension contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. Loteprednol etabonate is a white to off-white powder. Loteprednol ...
-
CLINICAL PHARMACOLOGYCorticosteroids inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte ...
-
INDICATIONS AND USAGELoteprednol etabonate ophthalmic suspension is indicated for the treatment of steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the ...
-
CONTRAINDICATIONSLoteprednol etabonate ophthalmic suspension, as with other ophthalmic corticosteroids, is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex ...
-
WARNINGSProlonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision, and in posterior subcapsular cataract formation. Steroids ...
-
PRECAUTIONSGeneral - For ophthalmic use only. The initial prescription and renewal of the medication order beyond 14 days should be made by a physician only after examination of the patient with the aid of ...
-
ADVERSE REACTIONSReactions associated with ophthalmic steroids include elevated intraocular pressure, which may be associated with optic nerve damage, visual acuity and field defects, posterior subcapsular ...
-
DOSAGE AND ADMINISTRATIONSHAKE VIGOROUSLY BEFORE USING. Steroid Responsive Disease Treatment: Apply one to two drops of loteprednol etabonate ophthalmic suspension into the conjunctival sac of the affected eye(s) four ...
-
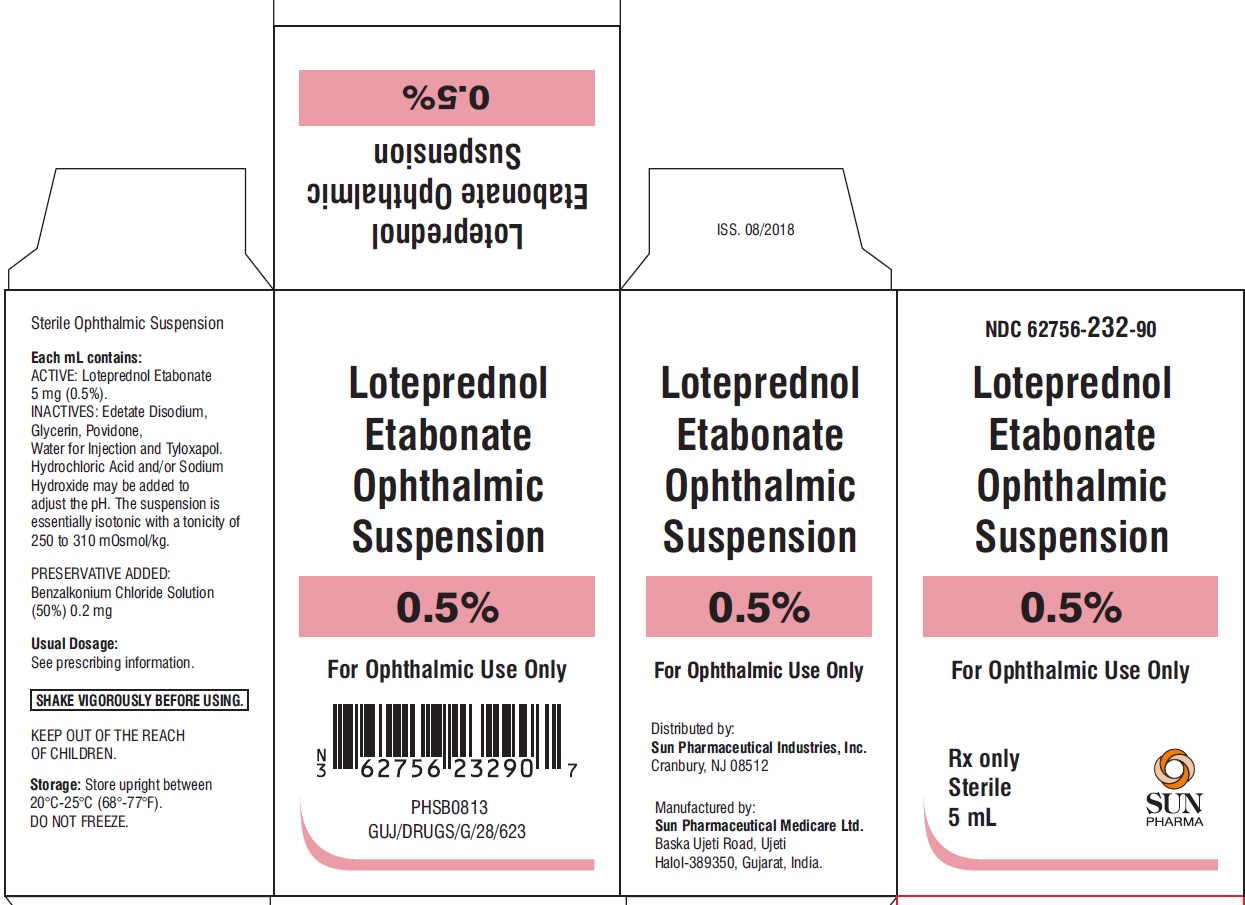
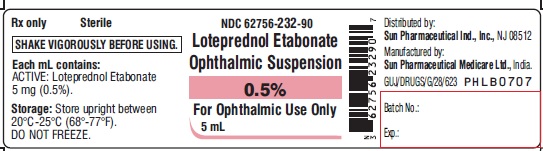
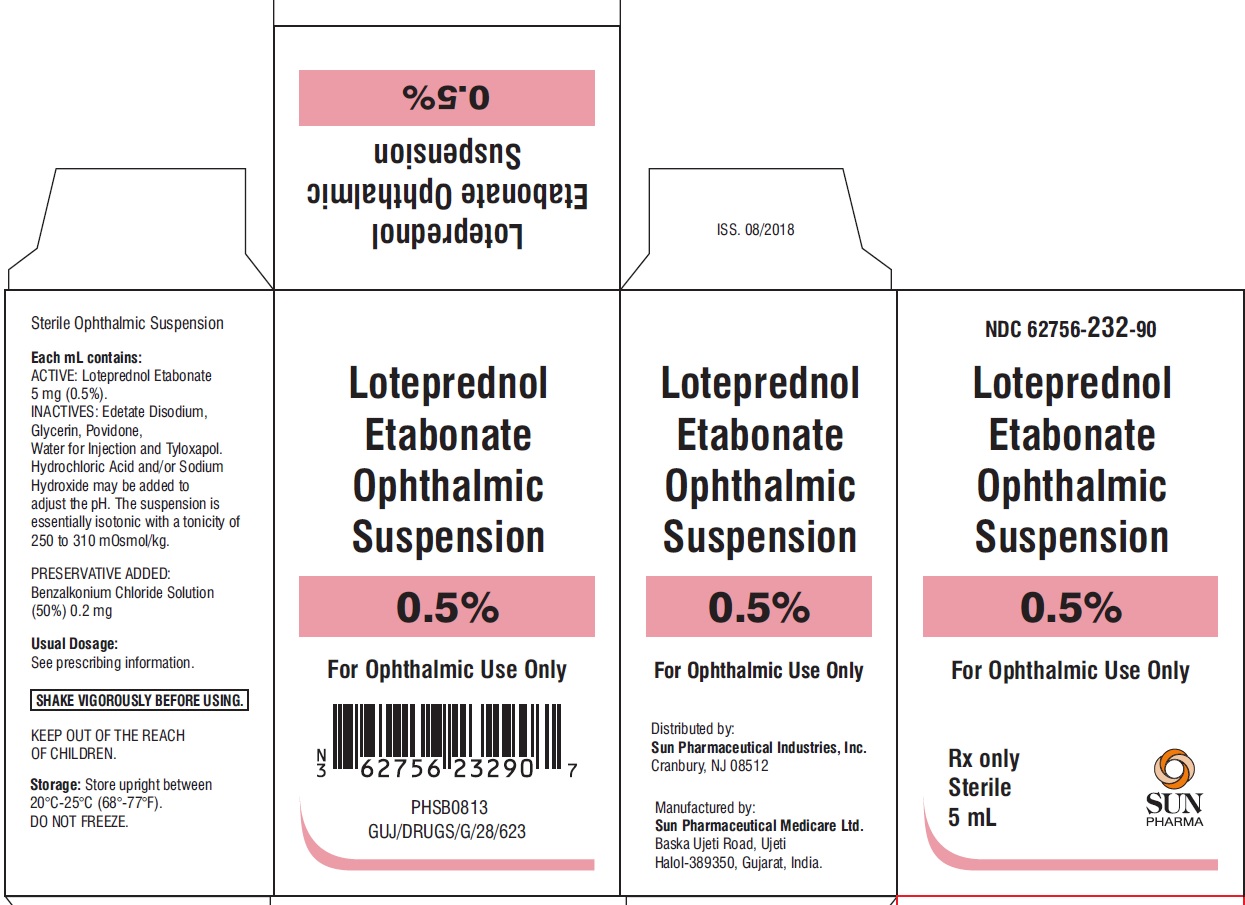
HOW SUPPLIEDLoteprednol etabonate ophthalmic suspension is supplied in a white opaque LDPE plastic dropper bottle with LDPE white opaque plug and HDPE pilfer-proof pink cap as follows: 5 mL (NDC ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-CartonNDC 62756-232-90 - Loteprednol Etabonate Ophthalmic Suspension 0.5% For Ophthalmic Use Only - Rx only Sterile 5 mL - SUN PHARMA
-
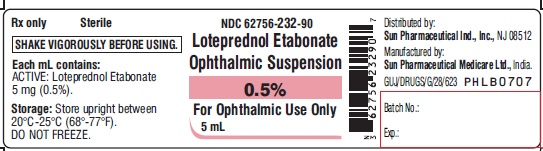
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-LabelNDC 62756-232-90 - Loteprednol Etabonate Ophthalmic Suspension 0.5% For Ophthalmic Use Only - 5 mL
-
INGREDIENTS AND APPEARANCEProduct Information