Label: DESMOPRESSIN ACETATE solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 62756-161-91 - Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 25, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
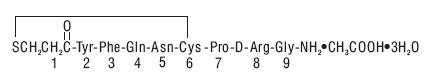
DESCRIPTION:Desmopressin nasal spray solution, USP 0.01% is a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It ...
-
CLINICAL PHARMACOLOGY:Desmopressin acetate is a synthetic analogue of the natural hormone arginine vasopressin. One mL (0.1 mg) of intranasal desmopressin acetate has an antidiuretic activity of about 400 IU; 10 mcg ...
-
INDICATIONS AND USAGE:Central Cranial Diabetes Insipidus: Desmopressin nasal spray solution, USP 0.01% is indicated as antidiuretic replacement therapy in the management of central cranial diabetes insipidus and for ...
-
CONTRAINDICATIONS:Desmopressin nasal spray solution 0.01% is contraindicated in individuals with known hypersensitivity to desmopressin acetate or to any of the components of desmopressin nasal spray solution ...
-
WARNINGS:For intranasal use only. Desmopressin nasal spray solution 0.01% should only be used in patients where orally administered formulations are not feasible. Very rare cases of hyponatremia have been ...
-
PRECAUTIONS:General: Intranasal desmopressin acetate at high dosage has infrequently produced a slight elevation of blood pressure, which disappeared with a reduction in dosage. The drug should be used ...
-
ADVERSE REACTIONS:Infrequently, high dosages of intranasal desmopressin acetate have produced transient headache and nausea. Nasal congestion, rhinitis and flushing have also been reported occasionally along with ...
-
OVERDOSAGE:Signs of overdose may include confusion, drowsiness, continuing headache, problems with passing urine and rapid weight gain due to fluid retention. (See WARNINGS.) In case of overdosage, the ...
-
DOSAGE AND ADMINISTRATION:Central Cranial Diabetes Insipidus: Desmopressin nasal spray solution 0.01% dosage must be determined for each individual patient and adjusted according to the diurnal pattern of response ...
-
HOW SUPPLIED:Desmopressin nasal spray solution, USP 0.01% is available in a 5 mL bottle with spray pump delivering 50 sprays of 10 mcg (NDC 62756-161-91). Storage: Store in refrigerator at 2°-8°C ...
-
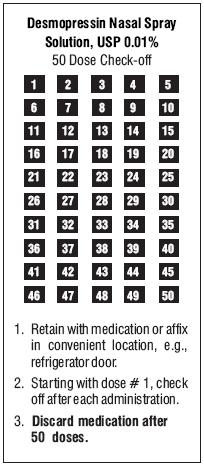
PATIENT INSTRUCTION GUIDEDesmopressin Nasal Spray Solution, USP 0.01% A better way to deliver desmopressin nasal spray solution, USP 0.01% Delivering desmopressin nasal spray solution, USP 0.01% more ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABELNDC 62756-161-91 - Desmopressin Nasal Spray Solution, USP 0.01% 0.1 mg/mL - 5 mL - REFRIGERATE - Rx only - FOR INTRANASAL USE ONLY. 50 doses of 10 mcg
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTONNDC 62756-161-91 - Desmopressin Nasal Spray Solution, USP 0.01% 0.1 mg/mL - (0.01 mg/Spray) FOR INTRANASAL USE ONLY. Rx only - 5 mL - 50 doses of 10 mcg - SUN PHARMA
-
INGREDIENTS AND APPEARANCEProduct Information