Mild-to-Moderate Alzheimer’s Disease
-
The effectiveness of rivastigmine tartrate as a treatment for Alzheimer's disease is demonstrated by the results of 2 randomized, double-blind ...
Mild-to-Moderate Alzheimer’s Disease
The effectiveness of rivastigmine tartrate as a treatment for Alzheimer's disease is demonstrated by the results of 2 randomized, double-blind, placebo-controlled clinical investigations (Study 1 and Study 2) in patients with Alzheimer's disease [diagnosed by NINCDS-ADRDA and DSM-IV criteria, Mini-Mental State Examination (MMSE) greater than or equal to10 and less than or equal to 26, and the Global Deterioration Scale (GDS)]. The mean age of patients participating in rivastigmine tartrate trials was 73 years with a range of 41 to 95. Approximately 59% of patients were women and 41% were men. The racial distribution was Caucasian 87%, Black 4%, and other races 9%.
In each study, the effectiveness of rivastigmine tartrate was evaluated using a dual outcome assessment strategy.
The ability of rivastigmine tartrate to improve cognitive performance was assessed with the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS-cog), a multi-item instrument that has been extensively validated in longitudinal cohorts of Alzheimer's disease patients. The ADAS-cog examines selected aspects of cognitive performance including elements of memory, orientation, attention, reasoning, language, and praxis. The ADAS-cog scoring range is from 0 to 70, with higher scores indicating greater cognitive impairment. Elderly normal adults may score as low as 0 or 1, but it is not unusual for non-demented adults to score slightly higher.
The patients recruited as participants in each study had mean scores on ADAS-cog of approximately 23 units, with a range from 1 to 61. Experience gained in longitudinal studies of ambulatory patients with mild-to-moderate Alzheimer's disease suggests that they gain 6 to 12 units a year on the ADAS-cog. Lesser degrees of change, however, are seen in patients with very mild or very advanced disease because the ADAS-cog is not uniformly sensitive to change over the course of the disease. The annualized rate of decline in the placebo patients participating in rivastigmine tartrate trials was approximately 3 to 8 units per year.
The ability of rivastigmine tartrate to produce an overall clinical effect was assessed using a Clinician's Interview-Based Impression of Change (CIBIC) that required the use of caregiver information, the CIBIC-Plus. The CIBIC-Plus is not a single instrument and is not a standardized instrument like the ADAS-cog. Clinical trials for investigational drugs have used a variety of CIBIC formats, each different in terms of depth and structure. As such, results from a CIBIC-Plus reflect clinical experience from the trial or trials in which it was used and cannot be compared directly with the results of CIBIC-Plus evaluations from other clinical trials. The CIBIC-Plus used in the rivastigmine tartrate trials was a structured instrument based on a comprehensive evaluation at baseline and subsequent time-points of 3 domains: patient cognition, behavior and functioning, including assessment of activities of daily living. It represents the assessment of a skilled clinician using validated scales based on his/her observation at interviews conducted separately with the patient and the caregiver familiar with the behavior of the patient over the interval rated. The CIBIC-Plus is scored as a 7-point categorical rating, ranging from a score of 1, indicating "markedly improved," to a score of 4, indicating "no change" to a score of 7, indicating "marked worsening." The CIBIC-Plus has not been systematically compared directly to assessments not using information from caregivers or other global methods.
U.S. 26-Week Study of Rivastigmine Tartrate in Mild-to-Moderate Alzheimer’s Disease (Study 1)
In a study of 26 weeks duration, 699 patients were randomized to either a dose range of 1 mg to 4 mg or 6 mg to 12 mg of rivastigmine tartrate per day or to placebo, each given in divided doses. The 26-week study was divided into a 12-week forced-dose titration phase and a 14-week maintenance phase. The patients in the active treatment arms of the study were maintained at their highest tolerated dose within the respective range.
Figure 1 illustrates the time course for the change from baseline in ADAS-cog scores for all 3 dose groups over the 26 weeks of the study. At 26 weeks of treatment, the mean differences in the ADAS-cog change scores for the rivastigmine tartrate-treated patients compared to the patients on placebo were 1.9 and 4.9 units for the 1 mg to 4 mg and 6 mg to 12 mg treatments, respectively. Both treatments were statistically significantly superior to placebo and the 6 mg to 12 mg per day range was significantly superior to the 1 mg to 4 mg per day range.
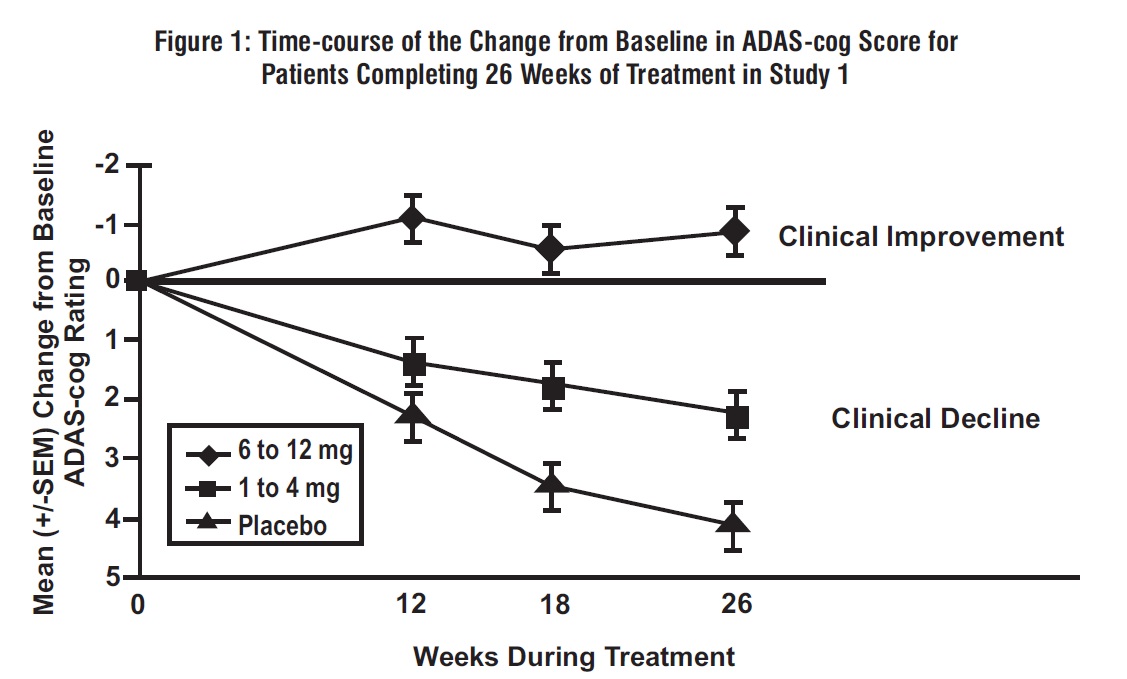
Figure 2 illustrates the cumulative percentages of patients from each of the 3 treatment groups who had attained at least the measure of improvement in ADAS-cog score shown on the x-axis. Three change scores, (7-point and 4-point reductions from baseline or no change in score) have been identified for illustrative purposes, and the percent of patients in each group achieving that result is shown in the inset table.
The curves demonstrate that both patients assigned to rivastigmine tartrate and placebo have a wide range of responses, but that the rivastigmine tartrate groups are more likely to show the greater improvements. A curve for an effective treatment would be shifted to the left of the curve for placebo, while an ineffective or deleterious treatment would be superimposed upon, or shifted to the right of the curve for placebo, respectively.
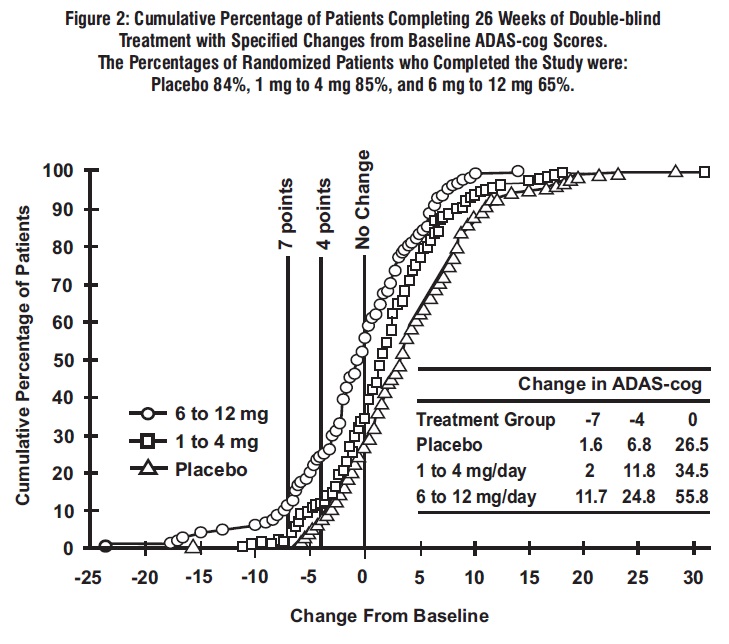
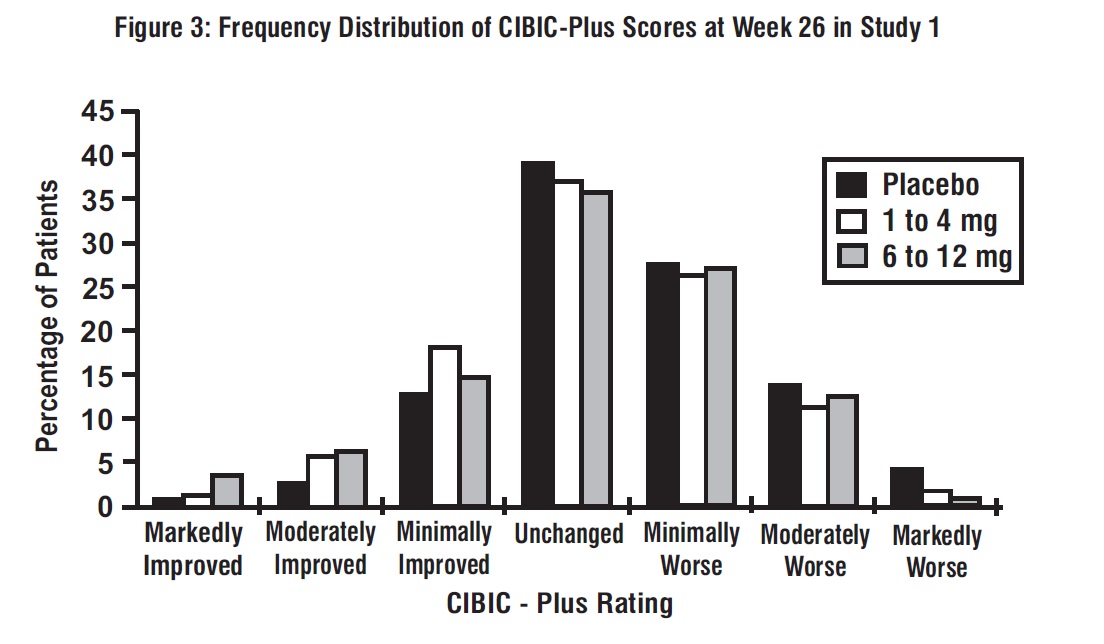
Figure 3 is a histogram of the frequency distribution of CIBIC-Plus scores attained by patients assigned to each of the 3 treatment groups who completed 26 weeks of treatment. The mean rivastigmine tartrate-placebo differences for these groups of patients in the mean rating of change from baseline were 0.32 units and 0.35 units for 1 mg to 4 mg and 6 mg to 12 mg of rivastigmine tartrate, respectively. The mean ratings for the 6 mg to 12 mg per day and 1 mg to 4 mg per day groups were statistically significantly superior to placebo. The differences between the 6 mg to 12 mg per day and the 1 mg to 4 mg per day groups were statistically significant.
Global 26-Week Study in Mild-to-Moderate Alzheimer’s Disease (Study 2)
In a second study of 26 weeks duration, 725 patients were randomized to either a dose range of 1 mg to 4 mg or 6 mg to 12 mg of rivastigmine tartrate per day or to placebo, each given in divided doses. The 26-week study was divided into a 12-week forced-dose titration phase and a 14-week maintenance phase. The patients in the active treatment arms of the study were maintained at their highest tolerated dose within the respective range.
Figure 4 illustrates the time course for the change from baseline in ADAS-cog scores for all 3 dose groups over the 26 weeks of the study. At 26 weeks of treatment, the mean differences in the ADAS-cog change scores for the rivastigmine tartrate-treated patients compared to the patients on placebo were 0.2 and 2.6 units for the 1 mg to 4 mg and 6 mg to 12 mg treatments, respectively. The 6 mg to 12 mg per day group was statistically significantly superior to placebo, as well as to the 1 mg to 4 mg per day group. The difference between the 1 mg to 4 mg per day group and placebo was not statistically significant.
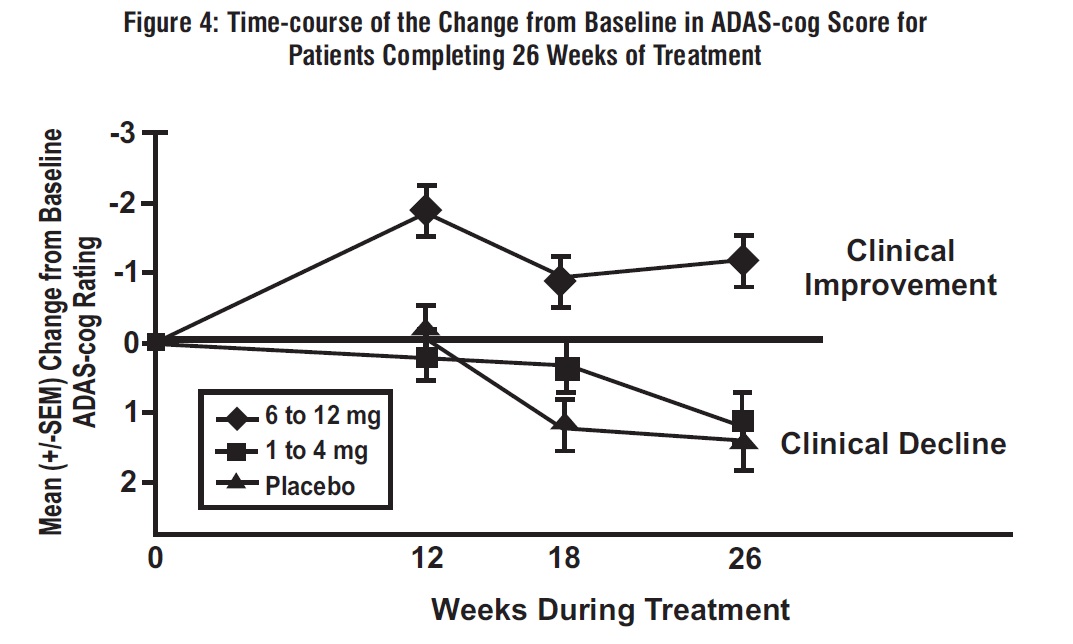
Figure 5 illustrates the cumulative percentages of patients from each of the 3 treatment groups who had attained at least the measure of improvement in ADAS-cog score shown on the x-axis. Similar to the U.S. 26-week study, the curves demonstrate that both patients assigned to rivastigmine tartrate and placebo have a wide range of responses, but that the 6 mg to 12 mg per day rivastigmine tartrate group is more likely to show the greater improvements.
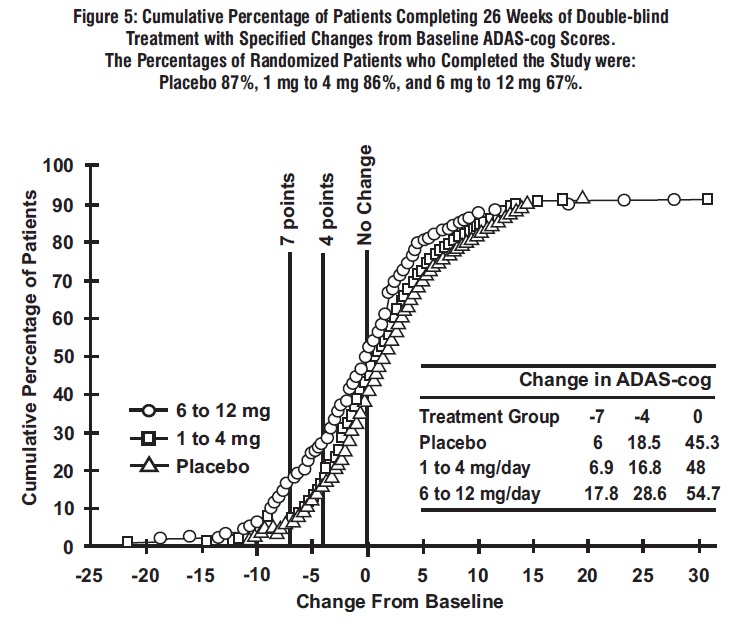
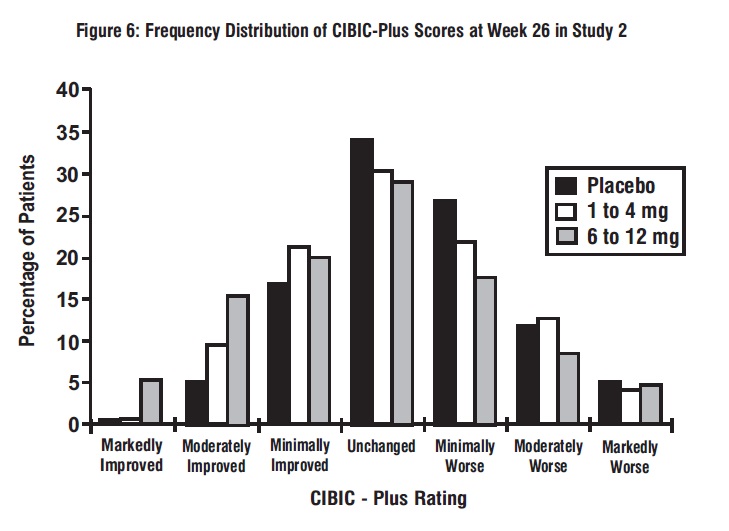
Figure 6 is a histogram of the frequency distribution of CIBIC-Plus scores attained by patients assigned to each of the 3 treatment groups who completed 26 weeks of treatment. The mean rivastigmine tartrate-placebo differences for these groups of patients for the mean rating of change from baseline were 0.14 units and 0.41 units for 1 mg to 4 mg and 6 mg to 12 mg of rivastigmine tartrate, respectively. The mean ratings for the 6 mg to 12 mg per day group were statistically significantly superior to placebo. The comparison of the mean ratings for the 1 mg to 4 mg per day group and placebo group was not statistically significant.
U.S. Fixed-Dose Study in Mild-to-Moderate Alzheimer’s Disease (Study 3)
In a study of 26 weeks duration, 702 patients were randomized to doses of 3 mg, 6 mg, or 9 mg per day of rivastigmine tartrate or to placebo, each given in divided doses. The fixed-dose study design, which included a 12-week forced-dose titration phase and a 14-week maintenance phase, led to a high dropout rate in the 9 mg per day group because of poor tolerability. At 26 weeks of treatment, significant differences were observed for the ADAS-cog mean change from baseline for the 9 mg per day and 6 mg per day groups, compared to placebo. No significant differences were observed between any of the rivastigmine tartrate-dose groups and placebo for the analysis of the CIBIC-Plus mean rating of change. Although no significant differences were observed between rivastigmine tartrate treatment groups, there was a trend toward numerical superiority with higher doses.
Mild-to-Moderate Parkinson’s Disease Dementia
International 24-Week Study (Study 4)
The effectiveness of rivastigmine tartrate as a treatment for dementia associated with Parkinson’s disease is demonstrated by the results of 1 randomized, double-blind, placebo-controlled clinical investigation in patients with mild-to-moderate dementia, with onset at least 2 years after the initial diagnosis of idiopathic Parkinson’s disease. The diagnosis of idiopathic Parkinson’s disease was based on the United Kingdom Parkinson’s Disease Society Brain Bank clinical criteria. The diagnosis of dementia was based on the criteria stipulated under the DSM-IV category “Dementia Due To Other General Medical Condition” (code 294.1x), but patients were not required to have a distinctive pattern of cognitive deficits as part of the dementia. Alternate causes of dementia were excluded by clinical history, physical and neurological examination, brain imaging, and relevant blood tests. Patients enrolled in the study had a MMSE score greater than or equal to 10 and less than or equal to 24 at entry. The mean age of patients participating in this trial was 72.7 years with a range of 50 to 91 years. Approximately, 35.1% of patients were women and 64.9% of patients were men. The racial distribution was 99.6% Caucasian and other races 0.4%.
This study used a dual outcome assessment strategy to evaluate the effectiveness of rivastigmine tartrate.
The ability of rivastigmine tartrate to improve cognitive performance was assessed with the ADAS-cog.
The ability of rivastigmine tartrate to produce an overall clinical effect was assessed using the Alzheimer’s Disease Cooperative Study – Clinician’s Global Impression of Change (ADCS-CGIC). The ADCS-CGIC is a more standardized form of CIBIC-Plus and is also scored as a 7-point categorical rating, ranging from a score of 1, indicating "markedly improved," to a score of 4, indicating "no change" to a score of 7, indicating "marked worsening".
In this study, 541 patients were randomized to a dose range of 3 mg to 12 mg of rivastigmine tartrate per day or to placebo in a ratio of 2:1, given in divided doses. The 24-week study was divided into a 16-week titration phase and an 8-week maintenance phase. The patients in the active treatment arm of the study were maintained at their highest tolerated dose within the specified dose range.
Figure 7 illustrates the time course for the change from baseline in ADAS-cog scores for both treatment groups over the 24-week study. At 24 weeks of treatment, the mean difference in the ADAS-cog change scores for the rivastigmine tartrate-treated patients compared to the patients on placebo was 3.8 points. This treatment difference was statistically significant in favor of rivastigmine tartrate when compared to placebo.
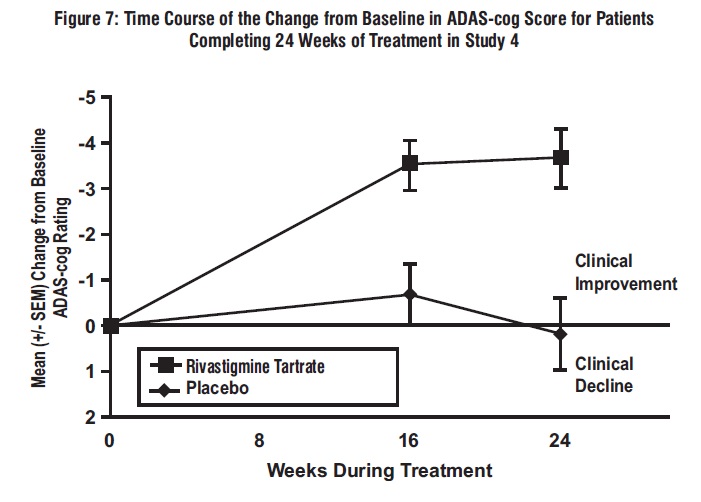
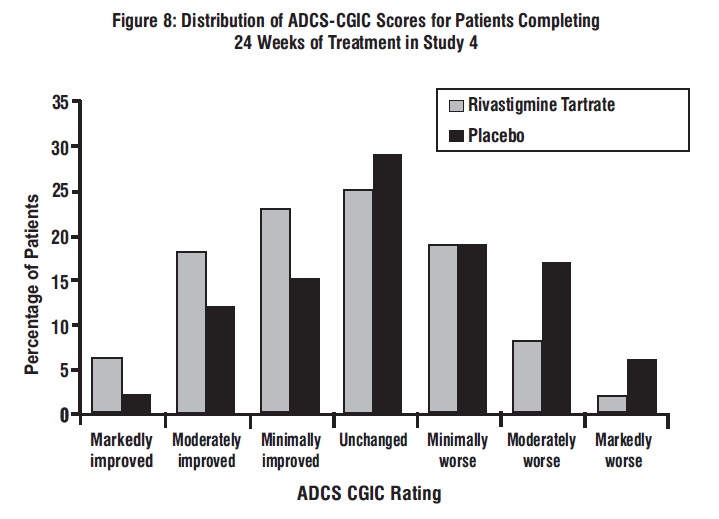
Figure 8 is a histogram of the distribution of patients’ scores on the ADCS-CGIC (Alzheimer’s Disease Cooperative Study - Clinician’s Global Impression of Change) at 24 weeks. The mean difference in change scores between the rivastigmine tartrate and placebo groups from baseline was 0.5 points. This difference was statistically significant in favor of rivastigmine tartrate treatment.
Patients’ age, gender, or race did not predict clinical outcome of rivastigmine tartrate treatment.
Close