Label: LIOTHYRONINE SODIUM tablet
- NDC Code(s): 62756-589-01, 62756-589-08, 62756-589-18, 62756-589-83, view more
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LIOTHYRONINE SODIUM TABLETS safely and effectively. See full prescribing information for LIOTHYRONINE SODIUM TABLETS. LIOTHYRONINE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
- Thyroid hormones, including liothyronine sodium tablets, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
- In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
- Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects [see Adverse Reactions (6), Drug Interactions (7.7), and Overdosage (10)].
-
1 INDICATIONS AND USAGE1.1 Hypothyroidism - Liothyronine sodium tablets are indicated as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Principles of Dosing - The dose of liothyronine sodium tablets for hypothyroidism or pituitary Thyroid-Stimulating Hormone (TSH) suppression depends on a variety of factors including ...
-
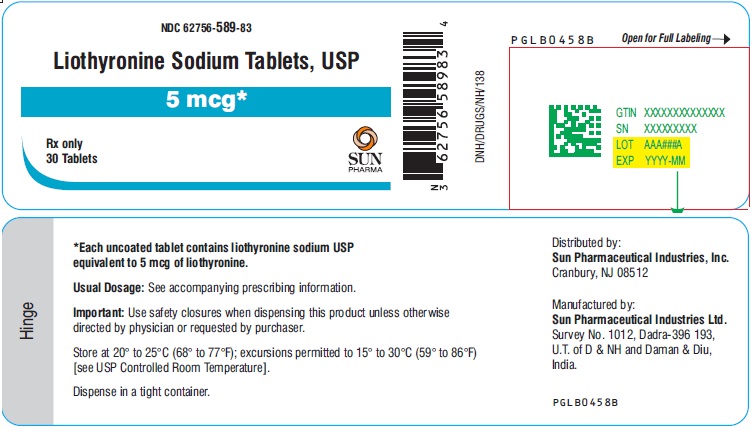
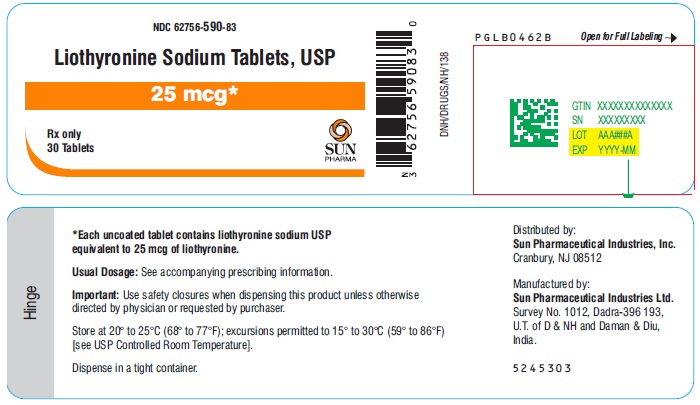
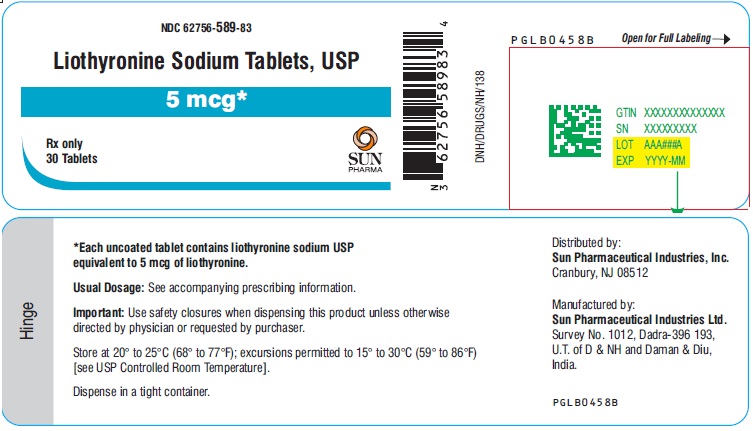
3 DOSAGE FORMS AND STRENGTHSTablets (circular, white to off-white) available as follows: • 5 mcg: debossed ‘589’ on one side and plain on other side - • 25 mcg: debossed ‘590’ on one side and breakline on other side - • 50 mcg ...
-
4 CONTRAINDICATIONSLiothyronine sodium tablets are contraindicated in patients with uncorrected adrenal insufficiency [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease - Overtreatment with thyroid hormone may cause an increase in heart rate, cardiac wall ...
-
6 ADVERSE REACTIONSAdverse reactions associated with liothyronine sodium therapy are primarily those of hyperthyroidism due to therapeutic overdosage [see Warnings and Precautions (5.4) and Overdosage (10)]. They ...
-
7 DRUG INTERACTIONS7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics - Many drugs can exert effects on thyroid hormone pharmacokinetics (e.g. absorption, synthesis, secretion, catabolism, protein binding ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Experience with liothyronine use in pregnant women, including data from postmarketing studies, have not reported increased rates of major birth defects or ...
-
10 OVERDOSAGEThe signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5.4) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral ...
-
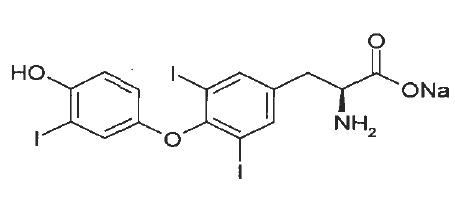
11 DESCRIPTIONLiothyronine sodium tablets, USP contain the active ingredient, liothyronine (L-triiodothyronine or LT3), a synthetic form of a thyroid hormone liothyronine in sodium salt form. It is chemically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and L-thyroxine (T4) diffuse into the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been performed to evaluate the carcinogenic potential, mutagenic potential or effects on fertility of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLiothyronine sodium tablets, USP are white to off-white, circular, uncoated tablets. They are supplied as follows: Liothyronine sodium tablets, containing 5 mcg liothyronine are debossed ‘589’ on ...
-
17 PATIENT COUNSELING INFORMATIONDosing and Administration - Instruct patients that liothyronine sodium tablets should only be taken as directed by their healthcare provider. Instruct patients to notify their healthcare provider ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 5 mcgNDC 62756-589-83 - Liothyronine Sodium Tablets, USP - 5 mcg - Rx only - 30 Tablets - SUN PHARMA
-
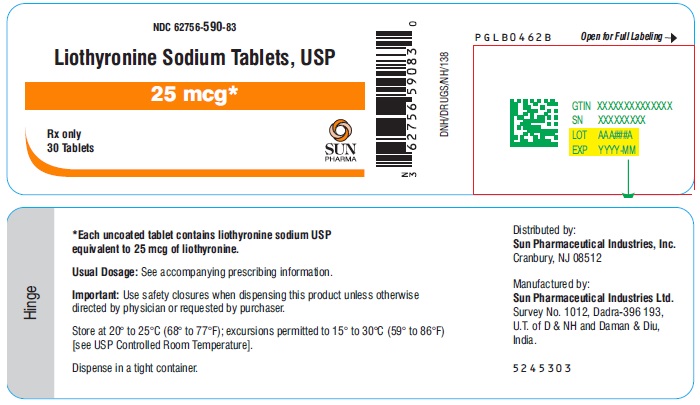
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 25 mcgNDC 62756-590-83 - Liothyronine Sodium Tablets, USP - 25 mcg - Rx only - 30 Tablets - SUN PHARMA
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 50 mcgNDC 62756-591-83 - Liothyronine Sodium Tablets, USP - 50 mcg - Rx only - 30 Tablets - SUN PHARMA
-
INGREDIENTS AND APPEARANCEProduct Information