Label: BICALUTAMIDE tablet
- NDC Code(s): 62559-890-30
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BICALUTAMIDE TABLETS safely and effectively. See full prescribing information for BICALUTAMIDE TABLETS. BICALUTAMIDE tablets, for ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGEBicalutamide tablets 50 mg daily is indicated for use in combination therapy with a luteinizing hormone-releasing hormone (LHRH) analog for the treatment of Stage D2 metastatic carcinoma of the ...
-
2. DOSAGE AND ADMINISTRATION2.1. Recommended Dose and Schedule - The recommended dose for bicalutamide therapy in combination with an LHRH analog is one 50 mg tablet once daily (morning or evening), with or without food ...
-
3. DOSAGE FORMS AND STRENGTHSBicalutamide Tablets USP 50 mg for oral administration.
-
4. CONTRAINDICATIONSBicalutamide tablets are contraindicated in: • Hypersensitivity - Bicalutamide tablets are contraindicated in any patient who has shown a hypersensitivity reaction to the drug or any of the ...
-
5. WARNINGS AND PRECAUTIONS5.1. Hepatitis - Cases of death or hospitalization due to severe liver injury (hepatic failure) have been reported postmarketing in association with the use of bicalutamide tablets ...
-
6. ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
7. DRUG INTERACTIONSClinical studies have not shown any drug interactions between bicalutamide and LHRH analogs (goserelin or leuprolide). There is no evidence that bicalutamide induces hepatic enzymes. In vitro ...
-
8. USE IN SPECIFIC POPULATIONS8.1. Pregnancy - Risk Summary - Bicalutamide tablets are contraindicated for use in pregnant women because it can cause fetal harm. Bicalutamide tablets are not indicated for use in females ...
-
10. OVERDOSAGELong-term clinical trials have been conducted with dosages up to 200 mg of bicalutamide daily and these dosages have been well tolerated. A single dose of bicalutamide that results in symptoms of ...
-
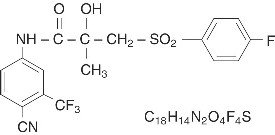
11. DESCRIPTIONBicalutamide Tablets USP contain 50 mg of bicalutamide USP, a non-steroidal androgen receptor inhibitor with no other known endocrine activity. The chemical name is propanamide, N [4 ...
-
12. CLINICAL PHARMACOLOGY12.1. Mechanism of Action - Bicalutamide is a non-steroidal androgen receptor inhibitor. It competitively inhibits the action of androgens by binding to cytosol androgen receptors in the target ...
-
13. NONCLINICAL TOXICOLOGY13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility - Two-year oral carcinogenicity studies were conducted in both male and female rats and mice at doses of 5, 15 or 75 mg/kg/day of ...
-
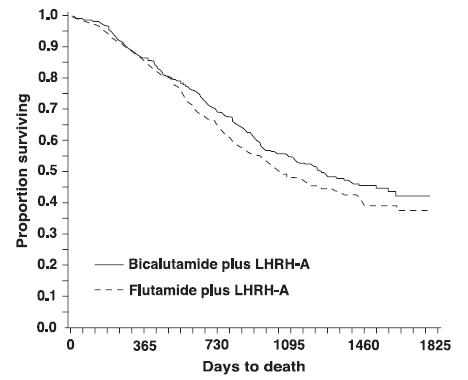
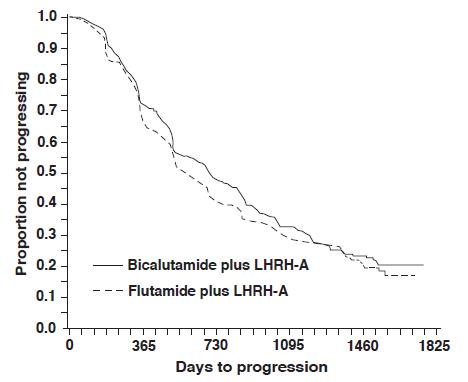
14. CLINICAL STUDIES14.1. Bicalutamide 50 mg Daily in Combination with an LHRH-A - In a multi-center, double-blind, controlled clinical trial, 813 patients with previously untreated advanced prostate cancer were ...
-
16. HOW SUPPLIED/STORAGE AND HANDLING White, film-coated tablets (identified on one side with "Cdx50" and on the reverse with the CASODEX logo) are supplied in unit dose bottles of 30 tablets (62559-890-30). 16.1. Storage and ...
-
17. PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Dose and Schedule: Inform patients that therapy with bicalutamide tablets and the LHRH analog should be started ...
-
PATIENT INFORMATION Bicalutamide (BYE-ka-LOO-ta-mide) Tablets - What are bicalutamide tablets? Bicalutamide tablets are a prescription medicine called an androgen receptor inhibitor, used in combination with ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC 62559-890-30 - Bicalutamide Tablets USP - 50 mg - Rx only - 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information