Label: MEXILETINE HYDROCHLORIDE capsule
- NDC Code(s): 62559-820-01, 62559-821-01, 62559-822-01
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Rx only
-
DESCRIPTION
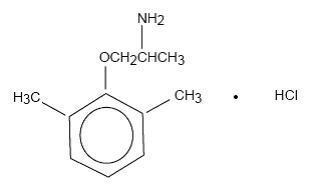
Mexiletine hydrochloride USP is an orally active antiarrhythmic agent. It is a white to off-white crystalline powder with slightly bitter taste, freely soluble in water and in alcohol. Mexiletine ...
-
CLINICAL PHARMACOLOGY Mechanism of Action - Mexiletine hydrochloride is a local anesthetic, antiarrhythmic agent, structurally similar to lidocaine, but orally active. In animal studies, mexiletine has been shown to ...
-
INDICATIONS AND USAGE Mexiletine Hydrochloride Capsules USP are indicated for the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, that, in the judgment of the physician, are ...
-
CONTRAINDICATIONS Mexiletine hydrochloride capsules are contraindicated in the presence of cardiogenic shock or preexisting second- or third-degree AV block (if no pacemaker is present).
-
WARNINGS BOXED WARNING - WARNINGS - Mortality - In the National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multicentered, randomized, double-blind study ...
-
PRECAUTIONS General - If a ventricular pacemaker is operative, patients with second or third degree heart block may be treated with mexiletine hydrochloride if continuously monitored. A limited number of ...
-
ADVERSE REACTIONS Mexiletine hydrochloride commonly produces reversible gastrointestinal and nervous system adverse reactions but is otherwise well tolerated. Mexiletine has been evaluated in 483 patients in one ...
-
OVERDOSAGE Clinical findings associated with mexiletine overdosage have included drowsiness, confusion, nausea, hypotension, sinus bradycardia, paresthesia, seizures, bundle branch block, AV heart block ...
-
DOSAGE AND ADMINISTRATION The dosage of mexiletine hydrochloride must be individualized on the basis of response and tolerance, both of which are dose-related. Administration with food or antacid is recommended. Initiate ...
-
HOW SUPPLIED Mexiletine Hydrochloride Capsules USP, 150 mg are white to yellow granular powder in a hard gelatin capsule with an opaque white cap and body, imprinted with ‘ANI’ on the cap and ‘820’ on the body ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Mexiletine Hydrochloride Capsules USP, 150 mg - NDC 62559-820-01 - TAKE WITH FOOD OR ANTACID. Rx only - 100 Capsules
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Mexiletine Hydrochloride Capsules USP, 200 mg - NDC 62559-821-01 - TAKE WITH FOOD OR ANTACID. Rx only - 100 Capsules
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Mexiletine Hydrochloride Capsules USP, 250 mg - NDC 62559-822-01 - TAKE WITH FOOD OR ANTACID. Rx only - 100 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information