Label: ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE tablet
- NDC Code(s): 62559-149-01, 62559-150-01
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
ESTROGENS INCREASE THE RISK OF ENDOMETRIAL CANCER
Close clinical surveillance of all women taking estrogens is important. Adequate diagnostic measures, including endometrial sampling when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal vaginal bleeding. There is no evidence that the use of "natural" estrogens results in a different endometrial risk profile than synthetic estrogens at equivalent estrogen doses. (See WARNINGS, Malignant Neoplasms, Endometrial Cancer.)
CARDIOVASCULAR AND OTHER RISKS
Estrogens with or without progestins should not be used for the prevention of cardiovascular disease. (See WARNINGS, Cardiovascular Disorders.)
The Women's Health Initiative (WHI) study reported increased risks of myocardial infarction, stroke, invasive breast cancer, pulmonary emboli, and deep vein thrombosis in postmenopausal women (50 to 79 years of age) during 5 years of treatment with oral conjugated estrogens (CE 0.625 mg) combined with medroxyprogesterone acetate (MPA 2.5 mg) relative to placebo. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
The Women's Health Initiative Memory Study (WHIMS), a substudy of WHI, reported increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with oral conjugated estrogens plus medroxyprogesterone acetate relative to placebo. It is unknown whether this finding applies to younger postmenopausal women or to women taking estrogen alone therapy. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
Other doses of oral conjugated estrogens with medroxyprogesterone acetate, and other combinations and dosage forms of estrogens and progestins were not studied in the WHI clinical trials and, in the absence of comparable data, these risks should be assumed to be similar. Because of these risks, estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Close -
DESCRIPTIONEsterified Estrogens and Methyltestosterone Tablets: Each dark green, capsule shaped, sugar-coated oral tablet imprinted with “1490” contains: 1.25 mg of Esterified Estrogens, USP and 2.5 mg of ...
-
CLINICAL PHARMACOLOGYEstrogens: Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating ...
-
INDICATIONS AND USAGEEsterified Estrogens and Methyltestosterone Full and Half-Strength Tablets are indicated in the: • Treatment of moderate to severe vasomotor symptoms associated with the menopause in those ...
-
CONTRAINDICATIONSEsterified Estrogens and Methyltestosterone Full and Half-Strength Tablets should not be used in women with any of the following conditions: 1. Undiagnosed abnormal genital bleeding. 2. Known ...
-
WARNINGSSee BOXED WARNINGS. Warnings Associated with Estrogens - Cardiovascular Disorders - Estrogen and estrogen/progestin therapy has been associated with an increased risk of cardiovascular ...
-
PRECAUTIONSGeneral Precautions Associated with Estrogens - Addition of a progestin when a woman has not had a hysterectomy: Studies of the addition of a progestin for 10 or more days of a cycle of estrogen ...
-
ADVERSE REACTIONSSee BOXED WARNINGS, WARNINGS and PRECAUTIONS. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be ...
-
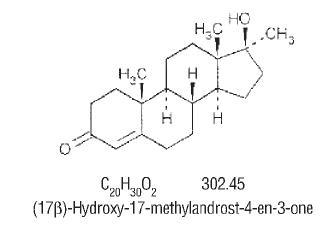
DRUG ABUSE AND DEPENDENCE Methyltestosterone is classified as a schedule III Controlled Substance under the Anabolic Steroids Act of 1990.
-
OVERDOSAGESerious ill effects have not been reported following acute ingestion of large doses of estrogen-containing drug products by young children. Overdosage of estrogen may cause nausea and vomiting ...
-
DOSAGE AND ADMINISTRATIONWhen estrogen is prescribed for a postmenopausal woman with a uterus, a progestin should also be initiated to reduce the risk of endometrial cancer. A woman without a uterus does not need ...
-
HOW SUPPLIEDEsterified Estrogens and Methyltestosterone Tablets (Imprinted “1490”) Bottles of 100 ……………………………………………………..NDC 62559-149-01 - Esterified Estrogens and Methyltestosterone Tablets (dark green ...
-
PATIENT INFORMATIONWHAT YOU SHOULD KNOW ABOUT ESTROGENS - Esterified Estrogens and Methyltestosterone Tablets, 1.25 mg/2.5 mg † and Esterified Estrogens and Methyltestosterone Tablets, 0.625 mg/1.25 mg ...
-
PRINCIPAL DISPLAY PANEL - 1.25 mg/2.5 mgEsterified Estrogens and Methyltestosterone Tablets, CIII - 1.25 mg/2.5 mg - NDC 62559-149-01 - Rx Only - 100 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.625 mg/1.25 mgEsterified Estrogens and Methyltestosterone Tablets, CIII - 0.625 mg/1.25 mg, Half-Strength - NDC 62559-150-01 - Rx Only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information