Label: MOXIFLOXACIN OPHTHALMIC SOLUTION solution/ drops
- NDC Code(s): 62332-505-03
- Packager: Alembic Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MOXIFLOXACIN OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for MOXIFLOXACIN OPHTHALMIC SOLUTION ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMoxifloxacin ophthalmic solution is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms: Corynebacterium species* Micrococcus ...
-
2 DOSAGE AND ADMINISTRATIONInstill one drop in the affected eye 3 times a day for 7 days. Moxifloxacin ophthalmic solution is for topical ophthalmic use.
-
3 DOSAGE FORMS AND STRENGTHSOphthalmic solution containing moxifloxacin 0.5%.
-
4 CONTRAINDICATIONSMoxifloxacin ophthalmic solution is contraindicated in patients with a history of hypersensitivity to moxifloxacin, to other quinolones, or to any of the components in this medication.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - In patients receiving systemically administered quinolones, including moxifloxacin, serious and occasionally fatal hypersensitivity (anaphylactic) reactions have ...
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical ...
-
7 DRUG INTERACTIONSDrug-drug interaction studies have not been conducted with moxifloxacin ophthalmic solution. In vitro studies indicate that moxifloxacin does not inhibit CYP3A4, CYP2D6, CYP2C9, CYP2C19, or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies with moxifloxacin ophthalmic solution in pregnant women to inform any drug-associated risks. Oral administration ...
-
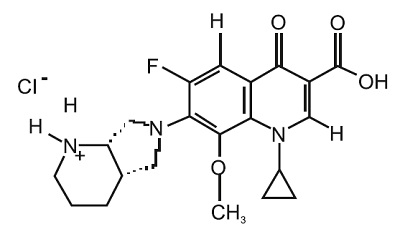
11 DESCRIPTIONMoxifloxacin ophthalmic solution USP 0.5% is a sterile solution for topical ophthalmic use. Moxifloxacin hydrochloride is an 8-methoxy fluoroquinolone anti-infective, with a diazabicyclononyl ring ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Moxifloxacin is a member of the fluoroquinolone class of anti-infective drugs (See 12.4 Microbiology). 12.3 Pharmacokinetics - Plasma concentrations of moxifloxacin ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals to determine the carcinogenic potential of moxifloxacin have not been performed ...
-
14 CLINICAL STUDIESIn two randomized, double-masked, multicenter, controlled clinical trials in which patients were dosed 3 times a day for 4 days, moxifloxacin ophthalmic solution produced clinical cures on day 5-6 ...
-
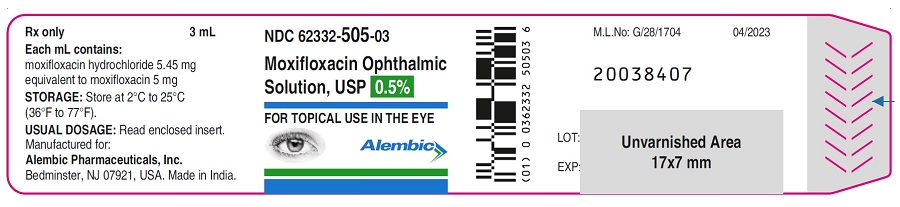
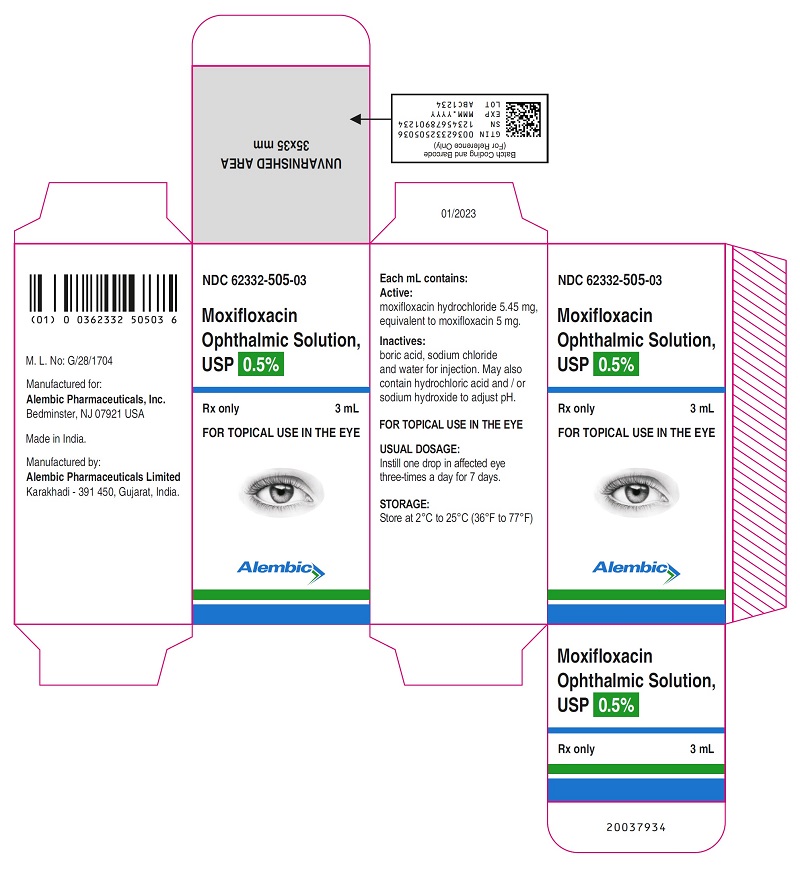
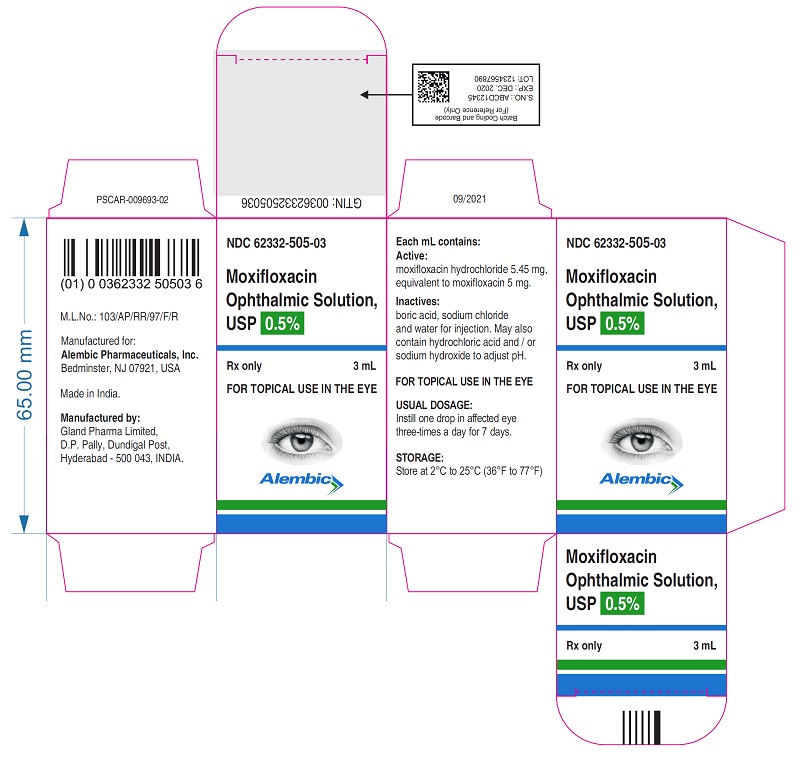
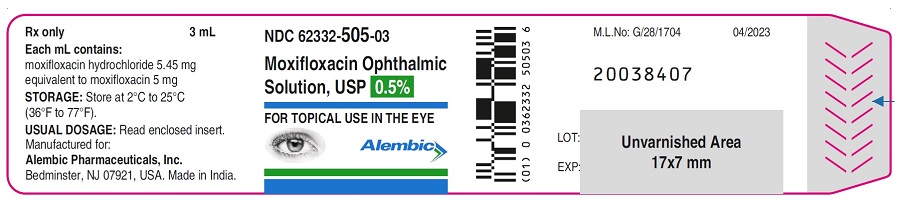
16 HOW SUPPLIED/STORAGE AND HANDLINGMoxifloxacin ophthalmic solution USP is supplied as a sterile ophthalmic solution in dispensing system consisting of a natural low density polyethylene bottle and dispensing plug and tan high ...
-
17 PATIENT COUNSELING INFORMATIONAvoid Contamination of the Product - Advise patients not to touch the dropper tip to any surface to avoid contaminating the contents. Avoid Contact Lens Wear - Advise patients not to wear ...
-
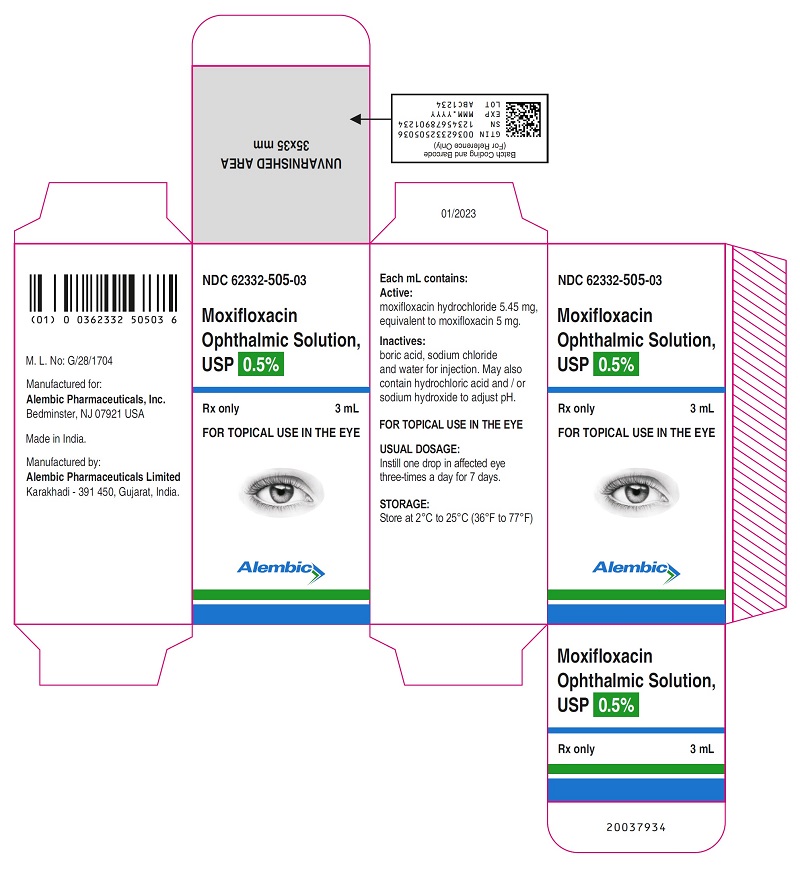
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - Bottle Label - Gland - PRINCIPAL DISPLAY PANEL - Carton Label - Gland - PRINCIPAL DISPLAY PANEL - Bottle Label - Alembic - PRINCIPAL DISPLAY PANEL - Carton Label ...
-
INGREDIENTS AND APPEARANCEProduct Information