Label: NADOLOL tablet
- NDC Code(s): 62332-402-30, 62332-402-31, 62332-402-91, 62332-403-30, view more
- Packager: Alembic Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
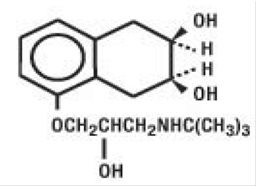
DESCRIPTIONNadolol, USP is a synthetic nonselective beta-adrenergic receptor blocking agent designated chemically as 1-(tert-butylamino)-3-[(5,6,7,8-tetrahydro-cis-6,7-dihydroxy-1-naphthyl)oxy]-2-propanol ...
-
CLINICAL PHARMACOLOGYNadolol is a nonselective beta-adrenergic receptor blocking agent. Clinical pharmacology studies have demonstrated beta-blocking activity by showing (1) reduction in heart rate and cardiac output ...
-
INDICATIONS AND USAGEAngina Pectoris - Nadolol tablets are indicated for the long-term management of patients with angina pectoris. Hypertension - Nadolol tablets are indicated for the treatment of hypertension, to ...
-
CONTRAINDICATIONSNadolol is contraindicated in bronchial asthma, sinus bradycardia and greater than first degree conduction block, cardiogenic shock, and overt cardiac failure (see WARNINGS).
-
WARNINGSCardiac Failure - Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by beta-blockade may precipitate ...
-
PRECAUTIONSImpaired Renal Function - Nadolol should be used with caution in patients with impaired renal function (see DOSAGE AND ADMINISTRATION). INFORMATION FOR PATIENTS - Interruption or ...
-
ADVERSE REACTIONSMost adverse effects have been mild and transient and have rarely required withdrawal of therapy. Cardiovascular - Bradycardia with heart rates of less than 60 beats per minute occurs commonly ...
-
OVERDOSAGENadolol can be removed from the general circulation by hemodialysis. In addition to gastric lavage, the following measures should be employed, as appropriate. In determining the duration of ...
-
DOSAGE AND ADMINISTRATIONDOSAGE MUST BE INDIVIDUALIZED. NADOLOL TABLETS MAY BE ADMINISTERED WITHOUT REGARD TO MEALS. Angina Pectoris - The usual initial dose is 40 mg nadolol once daily. Dosage may be gradually increased ...
-
HOW SUPPLIEDNadolol Tablets, USP are available containing 20 mg, 40 mg or 80 mg of nadolol, USP. The 20 mg tablets are light blue to blue color, round biconvex uncoated tablets with “L” and “19” debossed on ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 20 mgNDC 62332-402-30 - Nadolol - Tablets, USP - 20 mg - Alembic - Rx only 30 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 40 mgNDC 62332-403-30 - Nadolol - Tablets, USP - 40 mg - Alembic - Rx only 30 Tablets
-
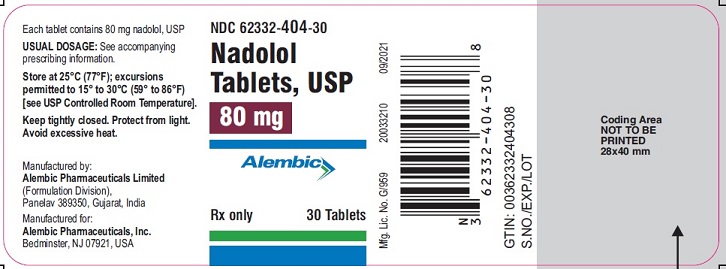
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 80 mgNDC 62332-404-30 - Nadolol - Tablets, USP - 80 mg - Alembic - Rx only 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information