Label: CANDESARTAN CILEXETIL tablet
- NDC Code(s): 62332-341-30, 62332-341-90, 62332-341-91, 62332-342-30, view more
- Packager: Alembic Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 30, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CANDESARTAN CILEXETIL TABLETS safely and effectively. See full prescribing information for CANDESARTAN CILEXETIL TABLETS ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
• When pregnancy is detected, discontinue candesartan cilexetil tablets as soon as possible [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Close
• Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
-
1 INDICATIONS AND USAGE1.1 Hypertension - Candesartan cilexetil tablets are indicated for the treatment of hypertension in adults and in children 1 to <17 years of age, to lower blood pressure. Lowering blood pressure ...
-
2 DOSAGE AND ADMINISTRATION2.1 Adult Hypertension - Dosage must be individualized. Blood pressure response is dose related over the range of 2 to 32 mg. The usual recommended starting dose of candesartan cilexetil tablet ...
-
3 DOSAGE FORMS AND STRENGTHS4 mg are white to off-white, round, biconvex, uncoated tablets debossed with ‘L168’ on one side and scoring on other side. 8 mg are light pink, round, biconvex, uncoated mottled tablets debossed ...
-
4 CONTRAINDICATIONSCandesartan cilexetil tablets are contraindicated in patients who are hypersensitive to candesartan. Do not co-administer aliskiren with candesartan cilexetil tablets in patients with diabetes ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Candesartan cilexetil tablets can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Agents Increasing Serum Potassium - Co-administration of candesartan cilexetil with potassium sparing diuretics, potassium supplements, potassium-containing salt substitutes or other drugs ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Candesartan cilexetil can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third ...
-
10 OVERDOSAGENo lethality was observed in acute toxicity studies in mice, rats, and dogs given single oral doses of up to 2000 mg/kg of candesartan cilexetil. In mice given single oral doses of the primary ...
-
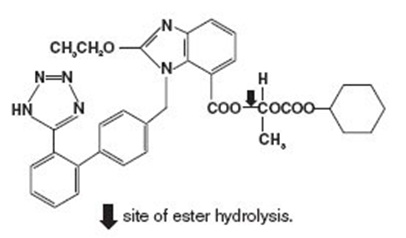
11 DESCRIPTIONCandesartan cilexetil, a prodrug, is hydrolyzed to candesartan during absorption from the gastrointestinal tract. Candesartan is a selective AT1 subtype angiotensin II ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There was no evidence of carcinogenicity when candesartan cilexetil was orally administered to mice and rats for up to 104 weeks at ...
-
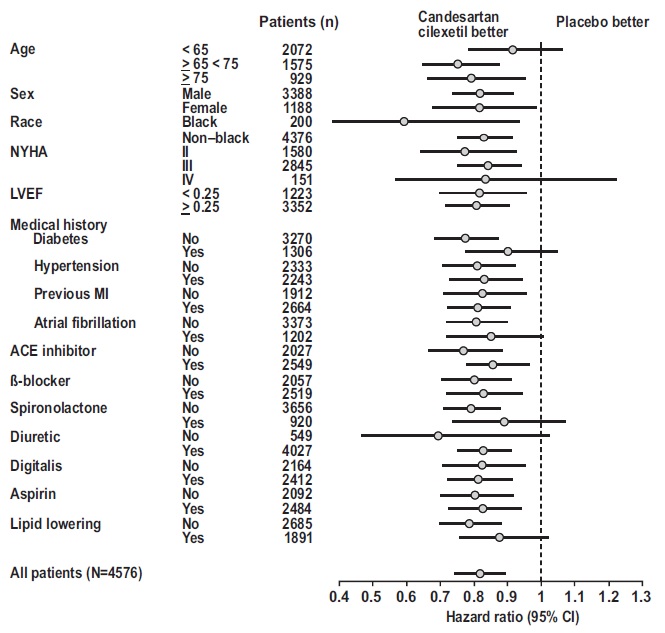
14 CLINICAL STUDIES14.1 Hypertension - Adult - The antihypertensive effects of candesartan cilexetil were examined in 14 placebo-controlled trials of 4- to 12-weeks duration, primarily at daily doses of 2 to 32 mg ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCandesartan cilexetil tablets USP, 4 mg are white to off-white, round, biconvex, uncoated tablets debossed with ‘L168’ on one side and scoring on other side. They are supplied as follows: NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patient to read FDA-approved patient labeling (Patient Information). Pregnancy - Advise female patients of childbearing age about the consequences of exposure to candesartan cilexetil ...
-
Patient InformationCandesartan Cilexetil (kan-de-sar-tan sye-lex-e-til) Tablets - Read the Patient Information that comes with candesartan cilexetil tablets before you start taking it and each time you get a ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 4 mgNDC 62332-341-30 - Candesartan Cilexetil - Tablets, USP - 4 mg - Rx only - 30 Tablets Alembic
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 8 mgNDC 62332-342-30 - Candesartan Cilexetil - Tablets, USP - 8 mg - Rx only - 30 Tablets - Alembic
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 16 mgNDC 62332-343-30 - Candesartan Cilexetil - Tablets, USP - 16 mg - Rx only - 30 Tablets - Alembic
-
INGREDIENTS AND APPEARANCEProduct Information