Label: CLONIDINE HYDROCHLORIDE tablet
- NDC Code(s): 62332-054-10, 62332-054-30, 62332-054-31, 62332-054-71, view more
- Packager: Alembic Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
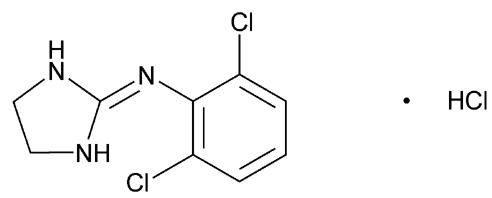
DESCRIPTIONClonidine hydrochloride is a centrally acting alpha-agonist hypotensive agent available as tablets for oral administration in three dosage strengths: 0.1 mg, 0.2 mg and 0.3 mg. The 0.1 mg tablet ...
-
CLINICAL PHARMACOLOGYClonidine stimulates alpha-adrenoreceptors in the brain stem. This action results in reduced sympathetic outflow from the central nervous system and in decreases in peripheral resistance, renal ...
-
INDICATIONS AND USAGEClonidine hydrochloride is indicated in the treatment of hypertension. Clonidine hydrochloride may be employed alone or concomitantly with other antihypertensive agents.
-
CONTRAINDICATIONSClonidine hydrochloride tablets USP should not be used in patients with known hypersensitivity to clonidine (see PRECAUTIONS).
-
WARNINGSWithdrawal - Patients should be instructed not to discontinue therapy without consulting their physician. Sudden cessation of clonidine treatment has, in some cases, resulted in symptoms such as ...
-
PRECAUTIONSGeneral - In patients who have developed localized contact sensitization to clonidine transdermal system, continuation of clonidine transdermal system or substitution of oral clonidine ...
-
ADVERSE REACTIONSMost adverse effects are mild and tend to diminish with continued therapy. The most frequent (which appear to be dose-related) are dry mouth, occurring in about 40 of 100 patients; drowsiness ...
-
OVERDOSAGEHypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, weakness, irritability and miosis ...
-
DOSAGE AND ADMINISTRATIONAdults - The dose of clonidine hydrochloride USP must be adjusted according to the patient's individual blood pressure response. The following is a general guide to its ...
-
HOW SUPPLIEDClonidine Hydrochloride Tablets, USP, are supplied as scored oval tablets containing 0.1 mg, 0.2 mg or 0.3 mg of clonidine hydrochloride. 0.1 mg tablets are light tan colored, oval shape ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 0.1 mgNDC 62332-054-30 - Clonidine - Hydrochloride - Tablets, USP - 0.1 mg - Rx only - 30 Tablets - Alembic
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 0.2 mgNDC 62332-055-30 - Clonidine - Hydrochloride - Tablets, USP - 0.2 mg - Rx only - 30 Tablets - Alembic
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 0.3 mgNDC 62332-056-30 - Clonidine - Hydrochloride - Tablets, USP - 0.3 mg - Rx only - 30 Tablets - Alembic
-
INGREDIENTS AND APPEARANCEProduct Information