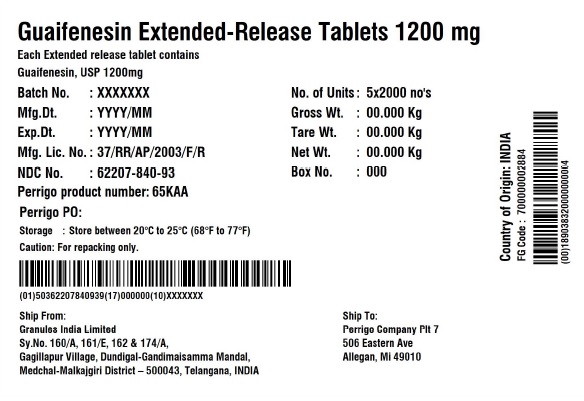
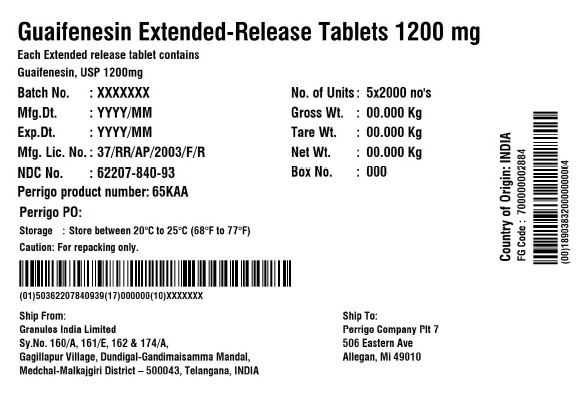
Label: GUAIFENESIN tablet, extended release
- NDC Code(s): 62207-839-72, 62207-840-71, 62207-840-93, 62207-840-99
- Packager: Granules India Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
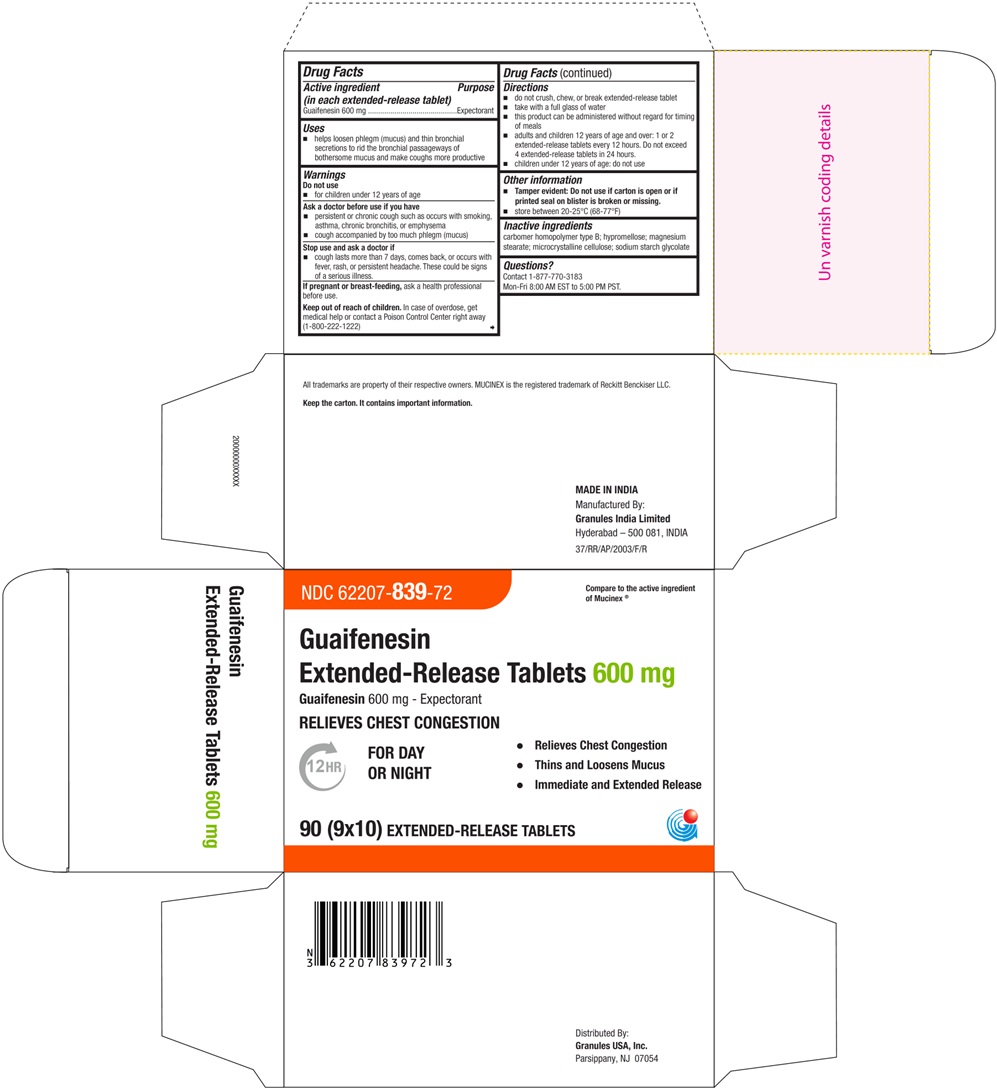
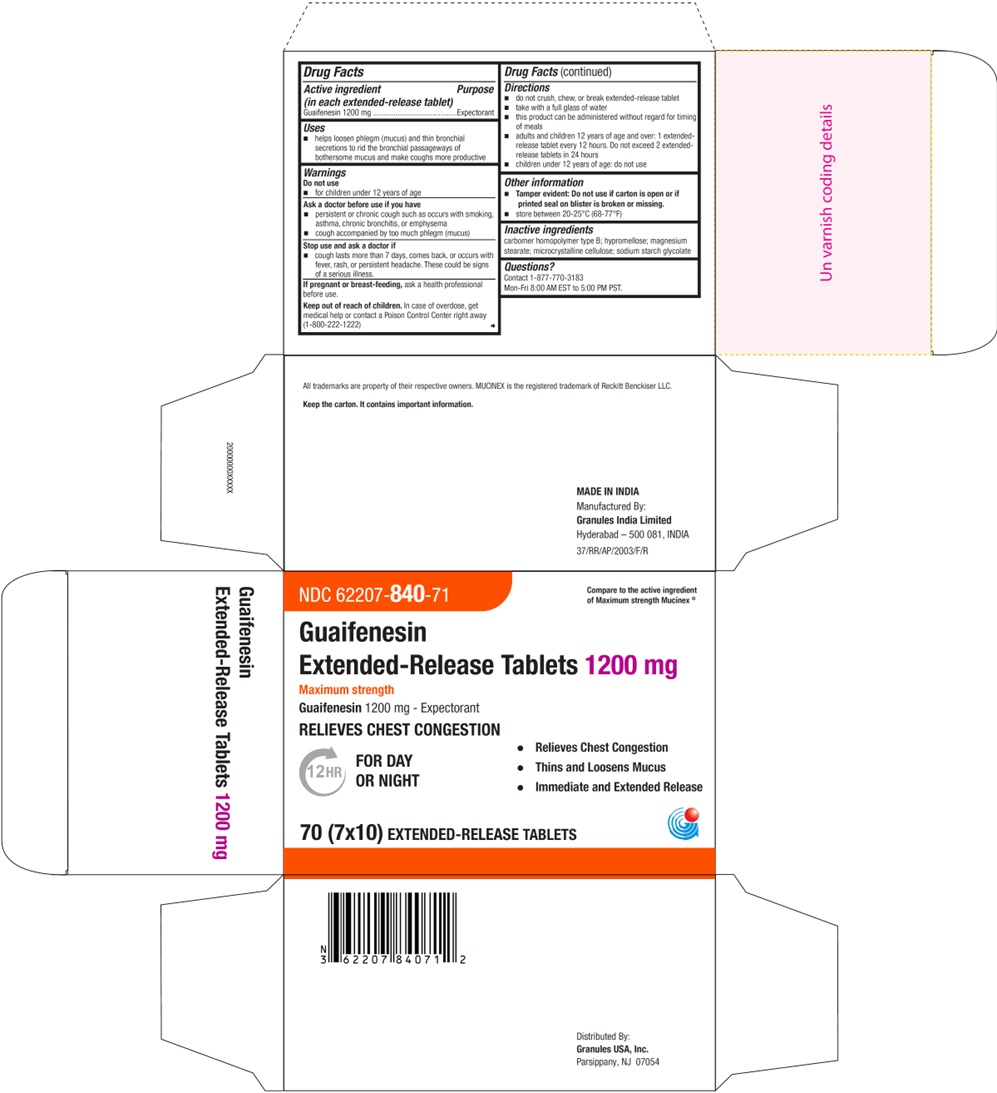
- Active ingredients (in each extended-release tablet)
- purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children
-
Directions
- do not crush, chew, or break extended-release tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years of age and over: 1 or 2 extended-release tablets every 12 hours. Do not exceed 4 extended-release tablets in 24 hours (For 600 mg)
- adults and children 12 years of age and over: 1 extended-release tablet every 12 hours. Do not exceed 2 extended-release tablets in 24 hours. (For 1200 mg)
- children under 12 years of age: do not use
- Other information
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL - 600 mg Blister Carton Label
NDC 62207- 839-72 Compare to the active ingredient
of Mucinex ®Guaifenesin
Extended-Release Tablets 600 mgGuaifenesin 600 mg - Expectorant
RELIEVES CHEST CONGESTION
12HR FOR DAY • Relieves Chest Congestion
OR NIGHT • Thins and Loosens Mucus
• Immediate and Extended Release90 (9 x 10) EXTENDED-RELEASE TABLETS
-
PRINCIPAL DISPLAY PANEL - 1200 mg Blister Carton Label
NDC 62207- 840-71 Compare to the active ingredient
of Mucinex®Guaifenesin
Extended-Release Tablets 1200 mgMaximum strength
Guaifenesin 1200 mg - Expectorant
RELIEVES CHEST CONGESTION12HR FOR DAY • Relieves Chest Congestion
OR NIGHT • Thins and Loosens Mucus
• Immediate and Extended Release70 (7 x 10) EXTENDED-RELEASE TABLETS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-839 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) Product Characteristics Color white Score no score Shape OVAL Size 16mm Flavor Imprint Code G;600 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-839-72 9 in 1 CARTON 12/18/2020 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213420 12/18/2020 GUAIFENESIN
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-840 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 1200 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape OVAL (Elliptical) Size 22mm Flavor Imprint Code G;1200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-840-71 7 in 1 CARTON 12/18/2020 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:62207-840-99 4200 in 1 BAG; Type 0: Not a Combination Product 12/18/2020 3 NDC:62207-840-93 10000 in 1 BOX; Type 0: Not a Combination Product 10/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213420 12/18/2020 Labeler - Granules India Ltd (915000087) Establishment Name Address ID/FEI Business Operations Ganules India Limited 918609236 analysis(62207-839, 62207-840) , label(62207-839, 62207-840) , manufacture(62207-839, 62207-840) , pack(62207-839, 62207-840)