Label: NIACIN tablet, extended release
- NDC Code(s): 62175-320-43, 62175-320-46, 62175-322-43, 62175-322-46
- Packager: Lannett Company, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NIACIN EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for NIACIN EXTENDED-RELEASE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETherapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to ...
-
2 DOSAGE AND ADMINISTRATION2.1 Initial Dosing - Niacin Extended-release Tablets, USP should be taken at bedtime, after a low-fat snack, and doses should be individualized according to patient response. Therapy with niacin ...
-
3 DOSAGE FORMS AND STRENGTHS• 500 mg unscored, red, round, film-coated, convex tablet debossed with “KU” on one side, “320” on the other side. • 1000 mg unscored, red, oval, film-coated, convex tablet debossed with “KU” on ...
-
4 CONTRAINDICATIONSNiacin extended-release tablets are contraindicated in the following conditions: • Active liver disease or unexplained persistent elevations in hepatic transaminases [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONSNiacin extended-release tablets preparations should not be substituted for equivalent doses of immediate-release (crystalline) niacin. For patients switching from immediate-release niacin to ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: • Mortality and Coronary Heart Disease Morbidity [see Warnings and Precautions (5.1)] • Skeletal ...
-
7 DRUG INTERACTIONS7.1 Statins - Caution should be used when prescribing niacin (≥1 gm/day) with statins as these drugs can increase risk of myopathy/rhabdomyolysis [see Warnings and Precautions (5) and Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Discontinue niacin extended-release tablets when pregnancy is recognized in patients receiving the drug for the treatment of hyperlipidemia. Assess the individual ...
-
10 OVERDOSAGESupportive measures should be undertaken in the event of an overdose.
-
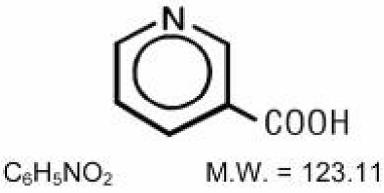
11 DESCRIPTIONNiacin Extended-release Tablets, USP (niacin tablet, film-coated extended-release), contain niacin, which at therapeutic doses is an antihyperlipidemic agent. Niacin (nicotinic acid, or ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism by which niacin alters lipid profiles has not been well defined. It may involve several actions including partial inhibition of release of free fatty ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility - Niacin administered to mice for a lifetime as a 1% solution in drinking water was not carcinogenic. The mice in this study ...
-
14 CLINICAL STUDIES14.1 Niacin Clinical Studies - Niacin’s ability to reduce mortality and the risk of definite, nonfatal myocardial infarction (MI) has been assessed in long-term studies. The Coronary Drug ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNiacin Extended-release Tablets, USP, 500 mg, are unscored, red, round, film-coated, convex tablets debossed with “KU” on one side, “320” on the other side. They are supplied as follows: Bottles ...
-
17 PATIENT COUNSELING INFORMATION17.1 Patient Counseling - Patients should be advised to adhere to their National Cholesterol Education Program (NCEP) recommended diet, a regular exercise program, and periodic testing of a ...
-
PATIENT PACKAGE INSERTDispense with Patient Information Leaflets available at: www.lannett.com/patient-info/niacin - PATIENT INFORMATION - Niacin (nye’ a sin) Extended-release Tablets, USP - for oral use - Read this ...
-
500 mg Bottle LabelPRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label - NDC 62175-320-46 - Niacin - Extended-release - Tablets, USP - 500 mg - Print Patient Informations at: www.lannett.com/patient-info/niacin - Rx ...
-
1000 mg Bottle LabelPRINCIPAL DISPLAY PANEL - 1,000 mg Tablet Bottle Label - NDC 62175-322-46 - Niacin - Extended-release - Tablets, USP - 1,000 mg - Print Patient Informations at: www.lannett.com/patient-info/niacin - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information