Label: OXYBUTYNIN CHLORIDE EXTENDED RELEASE- oxybutynin chloride tablet, extended release
- NDC Code(s): 62175-270-37, 62175-270-41, 62175-271-37, 62175-271-41, view more
- Packager: Lannett Company, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use OXYBUTYNIN CHLORIDE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for OXYBUTYNIN CHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEOxybutynin chloride extended-release tablets are a muscarinic antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and ...
-
2 DOSAGE AND ADMINISTRATIONOxybutynin chloride extended-release tablets must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed. Oxybutynin chloride extended-release tablets may be ...
-
3 DOSAGE FORMS AND STRENGTHSOxybutynin chloride extended-release tablets are available as 5, 10 and 15 mg tablets for oral use: 5 mg: White, round, biconvex tablet with "270" printed on one side and "KU" printed on the other ...
-
4 CONTRAINDICATIONSOxybutynin chloride extended-release tablets are contraindicated in patients with urinary retention, gastric retention and other severe decreased gastrointestinal motility conditions, uncontrolled ...
-
5 WARNINGS AND PRECAUTIONS5.1 Angioedema - Angioedema of the face, lips, tongue and/or larynx has been reported with oxybutynin. In some cases, angioedema occurred after the first dose. Angioedema associated with upper ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSThe concomitant use of oxybutynin with other anticholinergic drugs or with other agents which produce dry mouth, constipation, somnolence (drowsiness), and/or other anticholinergic-like effects ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on Oxybutynin chloride extended-release tablets use in pregnant women to evaluate for a drug-associated risk of major birth defects ...
-
10 OVERDOSAGEThe continuous release of oxybutynin from Oxybutynin chloride extended-release tablets should be considered in the treatment of overdosage. Patients should be monitored for at least 24 hours ...
-
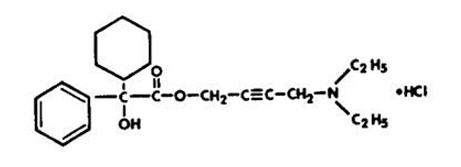
11 DESCRIPTIONOxybutynin chloride extended-release tablets are an antispasmodic, muscarinic antagonist. Each Oxybutynin chloride extended-release tablet contains 5 mg, 10 mg, or 15 mg of oxybutynin chloride ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Oxybutynin relaxes bladder smooth muscle. Oxybutynin chloride exerts a direct antispasmodic effect on smooth muscle and inhibits the muscarinic action of acetylcholine ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - A 24-month study in rats at dosages of oxybutynin chloride of 20, 80, and 160 mg/kg/day showed no evidence of ...
-
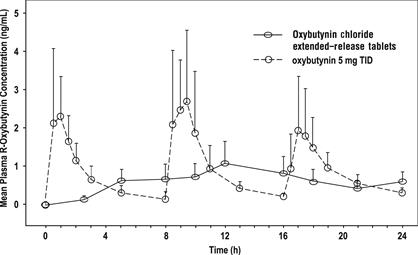
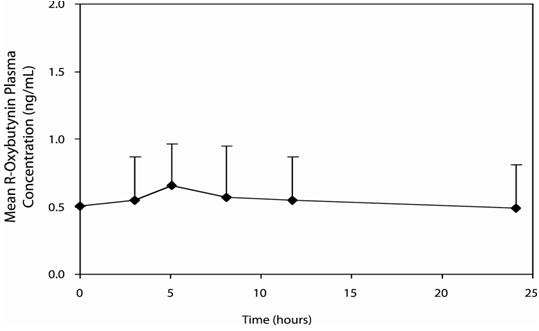
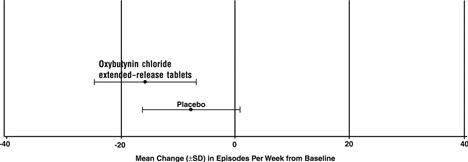
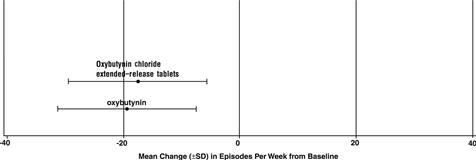
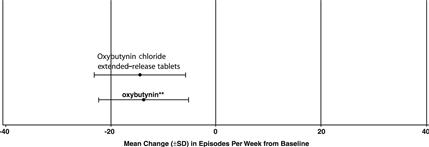
14 CLINICAL STUDIESOxybutynin chloride extended-release tablets were evaluated for the treatment of patients with overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency in three ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGOxybutynin chloride extended-release tablets, 5 mg are round, biconvex, white coated tablets imprinted in black ink with "270" on one side and "KU" on the other side. They are supplied as ...
-
17 PATIENT COUNSELING INFORMATIONPatients should be informed that oxybutynin may produce angioedema that could result in life threatening airway obstruction. Patients should be advised to promptly discontinue oxybutynin therapy ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Lannett Company, Inc., Philadelphia, PA 19136 - CIA72763P - Rev. 05/2023
-
PRINCIPAL DISPLAY PANEL - 5 mg 100 Tablet Bottle LabelNDC 62175-270-37 - 100 tablets - Oxybutynin chloride - Extended-release tablets - 5 mg - Rx only
-
PRINCIPAL DISPLAY PANEL - 5 mg 500 Tablet Bottle LabelNDC 62175-270-41 - 500 tablets - Oxybutynin chloride - Extended-release tablets - 5 mg - Rx only
-
PRINCIPAL DISPLAY PANEL - 10 mg 100 Tablet Bottle LabelNDC 62175-271-37 - 100 tablets - Oxybutynin chloride - Extended-release tablets - 10 mg - Rx only
-
PRINCIPAL DISPLAY PANEL - 10 mg 500 Tablet Bottle LabelNDC 62175-271-41 - 500 tablets - Oxybutynin chloride - Extended-release tablets - 10 mg - Rx only
-
PRINCIPAL DISPLAY PANEL - 15 mg 100 Tablet Bottle LabelNDC 62175-272-37 - 100 tablets - Oxybutynin chloride - Extended-release tablets - 15 mg - Rx only
-
PRINCIPAL DISPLAY PANEL - 15 mg 500 Tablet Bottle LabelNDC 62175-272-41 - 500 tablets - Oxybutynin chloride - Extended-release tablets - 15 mg - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information