Label: NIFEDIPINE tablet, film coated, extended release
- NDC Code(s): 62175-260-37, 62175-260-46, 62175-260-55, 62175-261-37, view more
- Packager: Lannett Company, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONNIFEdipine - Extended-release Tablets, USP - For Oral Use - Rx Only - CIA70797L ...
-
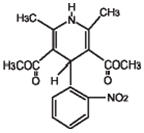
DESCRIPTIONNifedipine is a drug belonging to a class of pharmacological agents known as the calcium channel blockers. Nifedipine is 3,5-pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)- ...
-
CLINICAL PHARMACOLOGYNifedipine is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist) and inhibits the transmembrane influx of calcium ions into cardiac muscle and smooth muscle. The ...
-
INDICATIONS AND USAGEI. Vasospastic Angina - Nifedipine Extended-release Tablet is indicated for the management of vasospastic angina confirmed by any of the following criteria: 1) classical pattern of angina at rest ...
-
CONTRAINDICATIONSKnown hypersensitivity reaction to nifedipine.
-
WARNINGSExcessive Hypotension - Although in most angina patients the hypotensive effect of nifedipine is modest and well tolerated, occasional patients have had excessive and poorly tolerated ...
-
PRECAUTIONSGeneral-Hypotension - Because nifedipine decreases peripheral vascular resistance, careful monitoring of blood pressure during the initial administration and titration of nifedipine is suggested ...
-
ADVERSE EXPERIENCES
Over 1000 patients from both controlled and open trials with Nifedipine Extended-release Tablets in hypertension and angina were included in the evaluation of adverse experiences. All side effects ...
-
OVERDOSAGEExperience with nifedipine overdosage is limited. Generally, overdosage with nifedipine leading to pronounced hypotension calls for active cardiovascular support, including monitoring of ...
-
DOSAGE AND ADMINISTRATIONDosage must be adjusted according to each patient's needs. Therapy for either hypertension or angina should be initiated with 30 or 60 mg once daily. Nifedipine Extended-release Tablets should be ...
-
HOW SUPPLIEDNifedipine Extended-release Tablets 30 mg are round, biconvex, pink coated tablets imprinted with "KU 260" in black ink. They are supplied as follows: Bottles of 90 Tablets NDC ...
-
PRINCIPAL DISPLAY PANEL - 30MG 90CT90 Tablets NDC 62175-260-46 - Nifedipine - Extended Release - Tablets, USP
-
PRINCIPAL DISPLAY PANEL-30MG 100CT100 Tablets NDC 62175-260-37 - Nifedipine - Extended Release - Tablets, USP
-
PRINCIPAL DISPLAY PANEL-30MG 300CT300 Tablets NDC 62175-260-55 - Nifedipine - Extended Release - Tablets, USP
-
PRINCIPAL DISPLAY PANEL - 60MG 90CT90 Tablets NDC 62175-261-46 - Nifedipine - Extended Release - Tablets, USP
-
PRINCIPAL DISPLAY PANEL - 60MG 100CT100 Tablets NDC 62175-261-37 - Nifedipine - Extended ReleaseTablets, USP
-
PRINCIPAL DISPLAY PANEL- 60MG 300CT300 Tablets NDC 62175-261-55 - Nifedipine - Extended Release - Tablets, USP
-
PRINCIPAL DISPLAY PANEL - 90MG 30CT30 Tablets NDC 62175-262-32 - Nifedipine - Extended Release - Tablets, USP
-
PRINCIPAL DISPLAY PANEL-90MG 90CT90 Tablets NDC 62175-262-46 - Nifedipine - Extended Release - Tablets, USP
-
PRINCIPAL DISPLAY PANEL-90MG 100CT100 Tablets NDC 62175-262-37 - Nifedipine - Extended Release - Tablets, USP
-
INGREDIENTS AND APPEARANCEProduct Information