Label: NITROGLYCERIN film, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 62175-123-01, 62175-123-11, 62175-123-31, 62175-124-01, view more - Packager: Kremers Urban Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 1, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
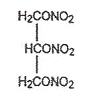
DESCRIPTIONNitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is: and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both ...
-
CLINICAL PHARMACOLOGYThe principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle and consequent dilatation of peripheral arteries and veins, especially the latter. Dilatation of the ...
-
INDICATIONS AND USAGETransdermal nitroglycerin is indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of transdermal nitroglycerin is not sufficiently rapid for this ...
-
CONTRAINDICATIONSAllergic reactions to organic nitrates are extremely rare, but they do occur. Nitroglycerin is contraindicated in patients who are allergic to it. Allergy to the adhesives used in nitroglycerin ...
-
WARNINGSAmplification of the vasodilatory effects of Nitroglycerin Transdermal System by phosphodiesterase inhibitors, e.g., sildenafil, can result in severe hypotension. The time course and dose ...
-
PRECAUTIONSGeneral: Severe hypotension, particularly with upright posture, may occur with even small doses of nitroglycerin, particularly in the elderly. This drug should, therefore, be used with caution ...
-
ADVERSE REACTIONSAdverse reactions to nitroglycerin are generally dose related, and almost all of these reactions are the result of nitroglycerin’s activity as a vasodilator. Headache, which may be severe, is the ...
-
OVERDOSAGEHemodynamic Effects: Nitroglycerin toxicity is generally mild. The estimated adult oral lethal dose of nitroglycerin is 200 mg to 1,200 mg. Infants may be more susceptible to toxicity from ...
-
DOSAGE AND ADMINISTRATIONThe suggested starting dose is between 0.2 mg/hr and 0.4 mg/hr. Doses between - 0.4 mg/hr and 0.8 mg/hr have shown continued effectiveness for 10 to 12 hours daily for at least 1 month (the ...
-
HOW SUPPLIEDNitroglycerin Transdermal System 0.2 mg/hr is a translucent, square patch with rounded corners imprinted with “nitroglycerin” and “0.2 mg/hr”, affixed to a translucent, peelable liner and is ...
-
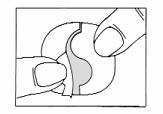
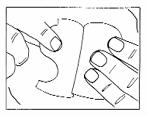
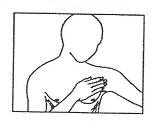
Patient InformationHow to use the Nitroglycerin Transdermal System for the prevention of angina - The Nitroglycerin Transdermal System is easy to use – it has a translucent plastic backing and a special adhesive ...
-
PRINCIPAL DISPLAY PANEL - 30 Patch CartonNDC 62175-123-01 - Contents: 30 systems - Nitroglycerin - Transdermal System - 0.2 mg/hr (10 cm2) Each 10 cm2 unit contains 20.8 mg of nitroglycerin in an - acrylic-based polymer adhesive with a ...
-
PRINCIPAL DISPLAY PANEL - 30 Patch CartonNDC 62175-124-01 - Contents: 30 systems - Nitroglycerin - Transdermal System - 0.4 mg/hr (18 cm2) Each 18 cm2 unit contains 37.4 mg of nitroglycerin in an - acrylic-based polymer adhesive with a ...
-
INGREDIENTS AND APPEARANCEProduct Information