Label: POTASSIUM CHLORIDE capsule, extended release
- NDC Code(s): 62037-559-01, 62037-559-05, 62037-560-01, 62037-560-05, view more
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use POTASSIUM CHLORIDE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for POTASSIUM CHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPotassium chloride extended-release capsules are indicated for the treatment and prophylaxis of hypokalemia in adults and children with or without metabolic alkalosis, in patients for whom dietary ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration and Monitoring - If serum potassium concentration is <2.5 mEq/L, use intravenous potassium instead of oral supplementation. Monitoring - Monitor serum potassium and adjust ...
-
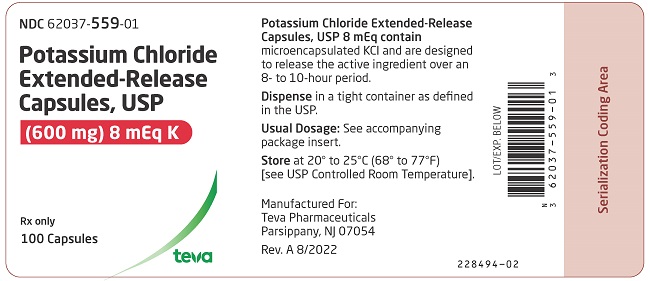
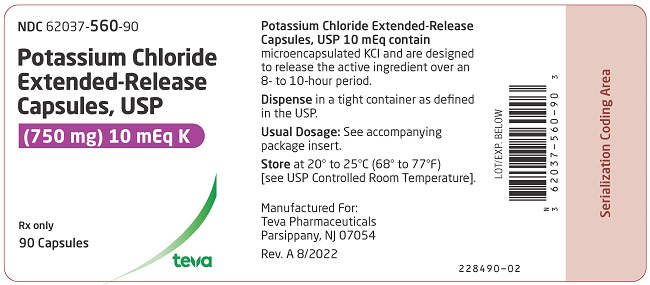
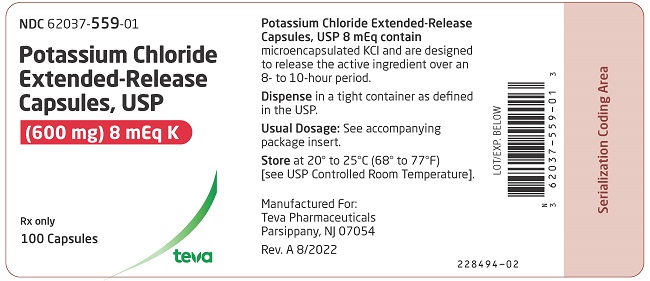
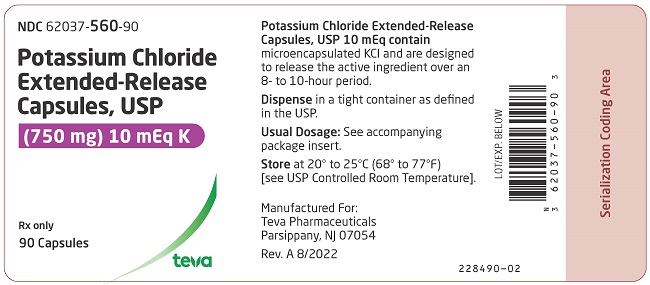
3 DOSAGE FORMS AND STRENGTHS600 mg (8 mEq): White opaque capsules imprinted with “Andrx” logo on the cap and “559” on the body - 750 mg (10 mEq ): Dark blue opaque capsules with “Andrx” logo on the cap and “560” on the ...
-
4 CONTRAINDICATIONSPotassium chloride extended-release capsules are contraindicated in patients on amiloride or triamterene.
-
5 WARNINGS AND PRECAUTIONS5.1 Gastrointestinal Adverse Reactions - Solid oral dosage forms of potassium chloride can produce ulcerative and/or stenotic lesions of the gastrointestinal tract, particularly if the drug is in ...
-
6 ADVERSE REACTIONSThe following adverse reactions have been identified with use of oral potassium salts. Because these reactions are reported voluntarily from a population of uncertain size, it is not always ...
-
7 DRUG INTERACTIONS7.1 Amiloride and Triamterene - Use with triamterene or amiloride can produce severe hyperkalemia. Concomitant use is contraindicated [see Contraindications (4)]. 7.2 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no human data related to use of potassium chloride extended-release capsules during pregnancy and animal reproductive studies have not been conducted ...
-
10 OVERDOSAGE10.1 Symptoms - The administration of oral potassium salts to persons with normal excretory mechanisms for potassium rarely causes serious hyperkalemia. However, if excretory mechanisms are ...
-
11 DESCRIPTIONPotassium chloride extended-release capsules USP, 8 mEq and 10 mEq are an oral dosage form of microencapsulated potassium chloride containing 600 mg and 750 mg of potassium chloride, USP ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The potassium ion (K+) is the principal intracellular cation of most body tissues. Potassium ions participate in a number of essential physiological processes ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPotassium chloride extended-release capsules USP, 8 mEq are white opaque capsules, imprinted with Andrx logo on the cap and 559 on the body, each containing 600 mg microencapsulated potassium ...
-
17 PATIENT COUNSELING INFORMATIONInform patients to take each dose with meals and with a full glass of water or other liquid. Advise patients seek medical attention if tarry stools or other evidence of gastrointestinal toxicity ...
-
PRINCIPAL DISPLAY PANELNDC 62037-559-01 - Potassium Chloride Extended-Release Capsules, USP - (600 mg) 8 mEq K - Rx only - 100 Capsules
-
PRINCIPAL DISPLAY PANELNDC 62037-560-90 - Potassium Chloride Extended-Release Capsules, USP - (750 mg) 10 mEq K - Rx only - 90 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information