Label: ACYCLOVIR tablet
- NDC Code(s): 61442-112-01, 61442-112-05, 61442-113-01, 61442-113-05
- Packager: Carlsbad Technology, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
DESCRIPTION
Acyclovir is a synthetic nucleoside analogue active against herpesviruses. Acyclovir tablets are formulations of an antiviral drug for oral administration. Each 800-mg tablet of acyclovir ...
-
VIROLOGY
Mechanism of Antiviral Action - Acyclovir is a synthetic purine nucleoside analogue with - in vitro and - in vivo inhibitory activity against herpes simplex virus types ...
-
CLINICAL PHARMACOLOGY SECTIONPharmacokinetics - The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster ...
-
INDICATIONS AND USAGE
Herpes Zoster Infections - Acyclovir is indicated for the acute treatment of herpes zoster (shingles). Genital Herpes - Acyclovir is indicated for the treatment of initial episodes and the ...
-
CONTRAINDICATIONS
Acyclovir is contraindicated for patients who develop hypersensitivity to acyclovir or valacyclovir.
-
WARNINGS
Acyclovir tablets are intended for oral ingestion only. Renal failure, in some cases resulting in death, has been observed with acyclovir therapy (see - ADVERSE REACTIONS: Observed ...
-
PRECAUTIONS
Dosage adjustment is recommended when administering acyclovir to patients with renal impairment (see - DOSAGE AND ADMINISTRATION). Caution should also be exercised when administering ...
-
ADVERSE REACTIONS
Herpes Simplex - Short-Term Administration: The most frequent adverse events reported during clinical trials of treatment of genital herpes with acyclovir 200 mg administered orally 5 times ...
-
OVERDOSAGE
Overdoses involving ingestion of up to 100 capsules (20 g) have been reported. Adverse events that have been reported in association with overdosage include agitation, coma, seizures, and ...
-
DOSAGE AND ADMINISTRATION
Acute Treatment of Herpes Zoster - 800 mg every 4 hours orally, 5 times daily for 7 to 10 days. Genital Herpes - Treatment of Initial Genital Herpes: 200 mg every 4 hours, 5 times daily ...
-
HOW SUPPLIEDAcyclovir Tablets, 800 mg (white to off-white, unscored, oval and engraved with “ ”) are supplied in bottles of 100 and 500. Bottle of 100 (NDC 61442-113-01) Bottle of 500 (NDC ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL - NDC 61442-112-01 - Acyclovir - Tablets USP - 400 mg - Rx Only - Carlsbad Technology, Inc. 100 Tablets
-
PRINCIPAL DISPLAY PANELNDC 61442-113-01 - Acyclovir - Tablets USP - 800 mg - Rx Only - Carlsbad Technology, Inc. 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information



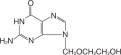
 ”) are supplied in bottles of 100 and 500.
”) are supplied in bottles of 100 and 500.
 ”) are supplied in bottles of 100 and 500.
”) are supplied in bottles of 100 and 500.

