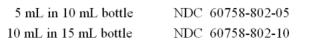Label: TIMOLOL MALEATE solution/ drops
- NDC Code(s): 60758-801-05, 60758-801-10, 60758-801-15, 60758-802-05, view more
- Packager: Pacific Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
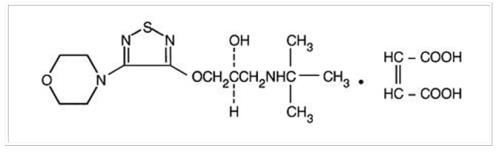
Timolol Maleate ophthalmic solution is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-(tert-Butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol ...
-
CLINICAL PHARMACOLOGY
Mechanism of Action: Timolol Maleate is a beta1 and beta2 (non-selective) adrenergic receptor blocking agent that does not have significant intrinsic sympathomimetic, direct myocardial depressant ...
-
INDICATIONS AND USAGE
Timolol Maleate ophthalmic solution is indicated in the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
-
CONTRAINDICATIONS
Timolol Maleate is contraindicated in patients with (1) bronchial asthma; (2) a history of bronchial asthma; (3) severe chronic obstructive pulmonary disease (see WARNINGS); (4) sinus bradycardia ...
-
WARNINGS
As with many topically applied ophthalmic drugs, this drug is absorbed systemically. The same adverse reactions found with systemic administration of beta-adrenergic blocking agents may occur ...
-
PRECAUTIONS
General - Because of potential effects of beta-adrenergic blocking agents on blood pressure and pulse, these agents should be used with caution in patients with cerebrovascular insufficiency ...
-
ADVERSE REACTIONS
The most frequently reported adverse experiences have been burning and stinging upon instillation (approximately one in eight patients). The following additional adverse experiences have been ...
-
OVERDOSAGE
There have been reports of inadvertent overdosage with Timolol Maleate ophthalmic solution resulting in systemic effects similar to those seen with systemic beta-adrenergic blocking agents such as ...
-
DOSAGE AND ADMINISTRATION
Timolol Maleate ophthalmic solution is available in concentrations of 0.25% and 0.5%. The usual starting dose is one drop of the 0.25% Timolol solution in the affected eye(s) twice a day. If the ...
-
HOW SUPPLIED
Timolol Maleate ophthalmic solution, USP is a clear, colorless to light yellow solution. Timolol Maleate ophthalmic solution, 0.25% timolol equivalent, is supplied sterile in opaque white LDPE ...
-
INFORMATION FOR PATIENTSInformation for the Patient - TIMOLOL MALEATE - ophthalmic solution, USP 0.25% and 0.5% sterile - Please follow these instructions carefully when using Timolol Maleate. Use Timolol Maleate ...
-
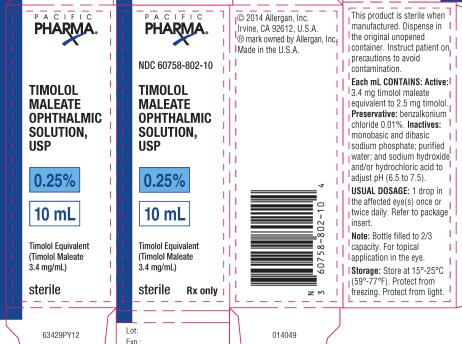
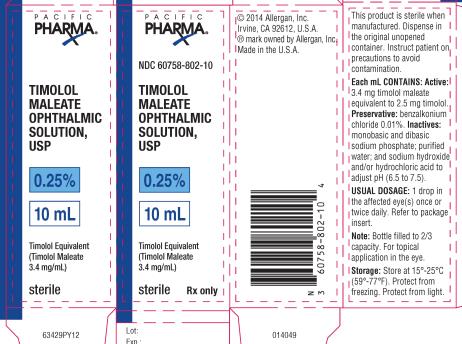
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 60758-802-10 - TIMOLOL - MALEATE - OPHTHALMIC - SOLUTION, USP - 0.25% 10 mL - Timolol Equivalent - (Timolol Maleate - 3.4 mg/mL) Sterile Rx only
-
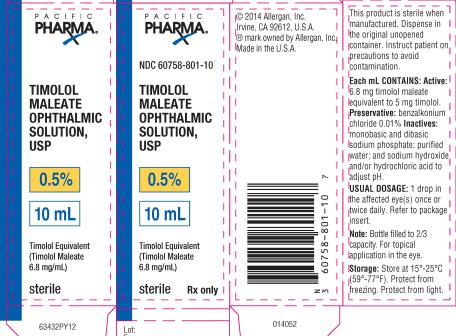
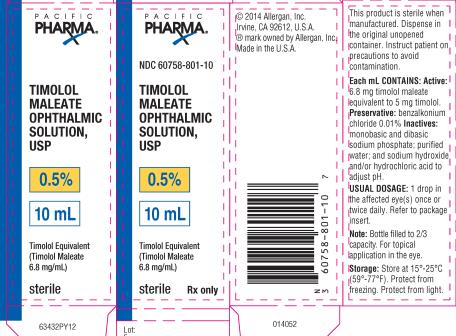
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 60758-801-10 - TIMOLOL - MALEATE - OPHTHALMIC - SOLUTION, USP - 0.5% 10 mL - Timolol Equivalent - (Timolol Maleate - 6.8 mg/mL) Sterile Rx only
-
INGREDIENTS AND APPEARANCEProduct Information

![Timolol Maleate ophthalmic solution is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-(tert-Butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol Maleate possesses an asymmetric carbon atom in its structure and is provided as the levo-isomer. The nominal optical rotation of Timolol Maleate is:](/dailymed/image.cfm?name=timolol-solution-01.jpg&setid=e16c33fd-aa86-49ad-a767-c79ade4a62c7)