Label: PREDNISOLONE ACETATE suspension/ drops
- NDC Code(s): 60758-119-05, 60758-119-10, 60758-119-15
- Packager: Pacific Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated March 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
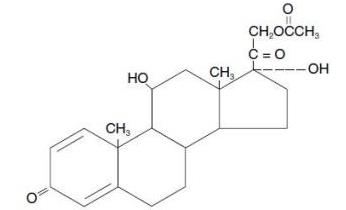
Prednisolone acetate ophthalmic suspension, USP 1% is a sterile, topical anti-inflammatory agent for ophthalmic use. Its chemical name is 11ß,17, 21-Trihydroxypregna-1,4-diene-3, 20-dione ...
-
CLINICAL PHARMACOLOGY
Prednisolone acetate is a glucocorticoid that, on the basis of weight, has 3 to 5 times the anti-inflammatory potency of hydrocortisone. Glucocorticoids inhibit the edema, fibrin deposition ...
-
INDICATIONS AND USAGE
Prednisolone acetate ophthalmic suspension 1% is indicated for the treatment of steroid-responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the ...
-
CONTRAINDICATIONS
Prednisolone acetate ophthalmic suspension 1% is contraindicated in acute untreated purulent ocular infections, in most viral diseases of the cornea and conjunctiva including epithelial herpes ...
-
WARNINGS
Prolonged use of corticosteroids may result in posterior subcapsular cataract formation and may increase intraocular pressure in susceptible individuals, resulting in glaucoma with damage to the ...
-
PRECAUTIONS
General - The initial prescription and renewal of the medication order beyond 20 milliliters of prednisolone acetate ophthalmic suspension 1% should be made by a physician only ...
-
ADVERSE REACTIONS
The following adverse reactions have been identified during use of prednisolone acetate ophthalmic suspension 1%. Because reactions are reported voluntarily from a population of uncertain size, it ...
-
OVERDOSAGE
Overdosage will not ordinarily cause acute problems. If accidentally ingested, drink fluids to dilute.
-
DOSAGE AND ADMINISTRATION
Shake well before using. Instill one to two drops into the conjunctival sac two to four times daily. During the initial 24 to 48 hours, the dosing frequency may be increased if necessary. Care ...
-
HOW SUPPLIED
Prednisolone acetate ophthalmic suspension, USP 1% is supplied sterile in opaque white LDPE plastic bottles with droppers with pink high impact polystyrene (HIPS) caps as follows: 5 mL in 10 mL ...
-
PRINCIPAL DISPLAY PANEL
NDC 60758-119-10 - PREDNISOLONE - ACETATE - ophthalmic - suspension, USP 1% 10 mL - sterile - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information