Label: LABETALOL HYDROCHLORIDE tablet, film coated
-
NDC Code(s):
60687-439-01,
60687-439-11,
60687-450-01,
60687-450-11, view more60687-461-01, 60687-461-11
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 68001-381, 68001-382, 68001-383
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Labetalol hydrochloride tablets, USP are an adrenergic receptor blocking agent that has both selective Alpha 1-adrenergic and nonselective beta-adrenergic receptor blocking actions in a single substance. Labetalol hydrochloride (HCl) is a racemate, chemically designated as 2-hydroxy-5-[1-hydroxy-2-[(1-methyl- 3-phenylpropyl)amino]ethyl]benzamide monohydrochloride and it has the following structure:
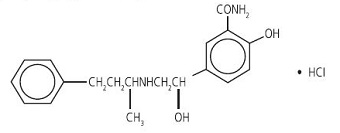
Labetalol hydrochloride has the molecular formula C 19H 24N 2O 3•HCl and a molecular weight of 364.87. It has two asymmetric centers and therefore exists as a molecular complex of two diastereoisomeric pairs. Dilevalol, the R,R' stereoisomer, makes up 25% of racemic labetalol.
Labetalol hydrochloride, USP is a white or almost white powder. It is sparingly soluble in water and in ethanol (96%), practically insoluble in ether and in methylene chloride.
Labetalol hydrochloride tablet, USP for oral administration contain 100 mg, 200 mg or 300 mg labetalol hydrochloride, USP. In addition, each tablet contains the following inactive ingredients: corn starch, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, sodium starch glycolate (botanical source: potato) and titanium dioxide. Additionally, 100 mg tablets contain D&C yellow #10 Aluminum lake, iron oxide yellow, iron oxide red and talc. 300 mg tablets contain FD&C blue #1 Aluminum lake, iron oxide yellow and talc.
-
CLINICAL PHARMACOLOGY
Labetalol hydrochloride combines both selective, competitive, alpha 1-adrenergic blocking and nonselective, competitive, beta-adrenergic blocking activity in a single substance. In man, the ratios of alpha- to beta-blockade have been estimated to be approximately 1:3 and 1:7 following oral and intravenous (IV) administration, respectively. Beta 2-agonist activity has been demonstrated in animals with minimal beta 1-agonist (ISA) activity detected. In animals, at doses greater than those required for alpha- or beta-adrenergic blockade, a membrane-stabilizing effect has been demonstrated.
Pharmacodynamics
The capacity of labetalol hydrochloride to block alpha receptors in man has been demonstrated by attenuation of the pressor effect of phenylephrine and by a significant reduction of the pressor response caused by immersing the hand in ice-cold water ("cold pressor test"). Labetalol hydrochloride's beta 1-receptor blockade in man was demonstrated by a small decrease in the resting heart rate, attenuation of tachycardia produced by isoproterenol or exercise, and by attenuation of the reflex tachycardia to the hypotension produced by amyl nitrite. Beta 2-receptor blockade was demonstrated by inhibition of the isoproterenol-induced fall in diastolic blood pressure. Both the alpha- and beta-blocking actions of orally administered labetalol hydrochloride contribute to a decrease in blood pressure in hypertensive patients. Labetalol hydrochloride consistently, in dose-related fashion, blunted increases in exercise-induced blood pressure and heart rate, and in their double product. The pulmonary circulation during exercise was not affected by labetalol hydrochloride dosing.
Single oral doses of labetalol hydrochloride administered to patients with coronary artery disease had no significant effect on sinus rate, intraventricular conduction, or QRS duration. The atrioventricular (A-V) conduction time was modestly prolonged in two of seven patients. In another study, intravenous (IV) labetalol hydrochloride slightly prolonged A-V nodal conduction time and atrial effective refractory period with only small changes in heart rate. The effects on A-V nodal refractoriness were inconsistent.
Labetalol hydrochloride produces dose-related falls in blood pressure without reflex tachycardia and without significant reduction in heart rate, presumably through a mixture of its alpha-blocking and beta-blocking effects. Hemodynamic effects are variable with small, non significant changes in cardiac output seen in some studies but not others, and small decreases in total peripheral resistance. Elevated plasma renins are reduced.
Doses of labetalol hydrochloride that controlled hypertension did not affect renal function in mildly to severely hypertensive patients with normal renal function.
Due to the alpha 1-receptor blocking activity of labetalol hydrochloride, blood pressure is lowered more in the standing than in the supine position and symptoms of postural hypotension (2%), including rare instances of syncope, can occur. Following oral administration, when postural hypotension has occurred, it has been transient and is uncommon when the recommended starting dose and titration increments are closely followed (see DOSAGE AND ADMINISTRATION). Symptomatic postural hypotension is most likely to occur 2 to 4 hours after a dose, especially following the use of large initial doses or upon large changes in dose.
The peak effects of single oral doses of labetalol hydrochloride occur within 2 to 4 hours. The duration of effect depends upon dose, lasting at least 8 hours following single oral doses of 100mg and more than 12 hours following single oral doses of 300 mg. The maximum, steady-state blood pressure response upon oral, twice-a-day dosing occurs within 24 to 72 hours.
The antihypertensive effect of labetalol has a linear correlation with the logarithm of labetalol plasma concentration and there is also a linear correlation between the reduction in exercise-induced tachycardia occurring at 2 hours after oral administration of labetalol hydrochloride and the logarithm of the plasma concentration.
About 70% of the maximum beta-blocking effect is present for 5 hours after the administration of a single oral dose of 400 mg with suggestion that about 40% remains at 8 hours.
The antianginal efficacy of labetalol hydrochloride has not been studied. In 37 patients with hypertension and coronary artery disease, labetalol hydrochloride did not increase the incidence or severity of angina attacks.
Exacerbation of angina and, in some cases, myocardial infarction and ventricular dysrhythmias have been reported after abrupt discontinuation of therapy with beta-adrenergic blocking agents in patients with coronary artery disease. Abrupt withdrawal of these agents in patients without coronary artery disease has resulted in transient symptoms, including tremulousness, sweating, palpitation, headache and malaise. Several mechanisms have been proposed to explain these phenomena, among them increased sensitivity to catecholamines because of increased numbers of beta receptors.
Although beta-adrenergic receptor blockade is useful in the treatment of angina and hypertension, there are also situations in which sympathetic stimulation is vital. For example, in patients with severely damaged hearts, adequate ventricular function may depend on sympathetic drive. Beta-adrenergic blockade may worsen A-V block by preventing the necessary facilitating effects of sympathetic activity on conduction. Beta 2-adrenergic blockade results in passive bronchial constriction by interfering with endogenous adrenergic bronchodilator activity in patients subject to bronchospasm and it may also interfere with exogenous bronchodilators in such patients.
Pharmacokinetics and Metabolism
Labetalol hydrochloride is completely absorbed from the gastrointestinal tract with peak plasma levels occurring 1 to 2 hours after oral administration. The relative bioavailability of labetalol hydrochloride tablets compared to an oral solution is 100%. The absolute bioavailability (fraction of drug reaching systemic circulation) of labetalol when compared to an intravenous infusion is 25%; this is due to extensive "first-pass" metabolism. Despite "first-pass" metabolism, there is a linear relationship between oral doses of 100 mg to 3000 mg and peak plasma levels. The absolute bioavailability of labetalol is increased when administered with food.
The plasma half-life of labetalol following oral administration is about 6 to 8 hours. Steady-state plasma levels of labetalol during repetitive dosing are reached by about the third day of dosing. Inpatients with decreased hepatic or renal function, the elimination half-life of labetalol is not altered; however, the relative bioavailability in hepatically impaired patients is increased due to decreased "first-pass" metabolism.
The metabolism of labetalol is mainly through conjugation to glucuronide metabolites. These metabolites are present in plasma and are excreted in the urine and, via the bile, into the feces. Approximately 55% to 60% of a dose appears in the urine as conjugates or unchanged labetalol within the first 24 hours of dosing.
Labetalol has been shown to cross the placental barrier in humans. Only negligible amounts of the drug crossed the blood-brain barrier in animal studies. Labetalol is approximately 50% protein bound. Neither hemodialysis nor peritoneal dialysis removes a significant amount of labetalol hydrochloride from the general circulation (less than 1%).
Elderly Patients
Some pharmacokinetic studies indicate that the elimination of labetalol is reduced in elderly patients. Therefore, although elderly patients may initiate therapy at the currently recommended dosage of 100 mg b.i.d., elderly patients will generally require lower maintenance dosages than nonelderly patients. - INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Labetalol hydrochloride is contraindicated in bronchial asthma, overt cardiac failure, greater-than-first-degree heart block, cardiogenic shock, severe bradycardia, other conditions associated with severe and prolonged hypotension and in patients with a history of hypersensitivity to any component of the product (see WARNINGS).
Beta-blockers, even those with apparent cardio selectivity, should not be used in patients with a history of obstructive airway disease, including asthma.
-
WARNINGS
Hepatic Injury
Severe hepatocellular injury, confirmed by rechallenge in at least one case, occurs rarely with labetalol therapy. The hepatic injury is usually reversible, but hepatic necrosis and death have been reported. Injury has occurred after both short- and long-term treatment and may be slowly progressive despite minimal symptomatology. Similar hepatic events have been reported with a related research compound, dilevalol hydrochloride, including two deaths. Dilevalol hydrochloride is one of the four isomers of labetalol hydrochloride. Thus, for patients taking labetalol, periodic determination of suitable hepatic laboratory tests would be appropriate. Appropriate laboratory testing should be done at the first symptom or sign of liver dysfunction (e.g. pruritus, dark urine, persistent anorexia, jaundice, right upper quadrant tenderness, or unexplained "flu-like" symptoms). If the patient has laboratory evidence of liver injury or jaundice, labetalol should be stopped and not restarted.
Cardiac Failure
Sympathetic stimulation is a vital component supporting circulatory function in congestive heart failure. Beta-blockade carries a potential hazard of further depressing myocardial contractility and precipitating more severe failure. Although beta-blockers should be avoided in overt congestive heart failure, if necessary, labetalol hydrochloride can be used with caution in patients with a history of heart failure who are well compensated. Congestive heart failure has been observed in patients receiving labetalol hydrochloride. Labetalol hydrochloride does not abolish the inotropic action of digitalis on heart muscle.
In Patients Without a History of Cardiac Failure
In patients with latent cardiac insufficiency, continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first sign or symptom of impending cardiac failure, patients should be fully digitalized and/or be given a diuretic and the response should be observed closely. If cardiac failure continues, despite adequate digitalization and diuretic, therapy with labetalol hydrochloride tablets should be withdrawn (gradually, if possible).
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal
Angina pectoris has not been reported upon labetalol hydrochloride discontinuation. However, hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing chronically administered labetalol hydrochloride tablets, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 to 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, therapy with labetalol hydrochloride tablet administration should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue therapy with labetalol hydrochloride tablets abruptly in patients being treated for hypertension.
Non allergic bronchospasm (e.g. chronic bronchitis and emphysema) patients with bronchospastic disease should, in general, not receive beta-blockers. Labetalol hydrochloride tablets may be used with caution, however, in patients who do not respond to, or cannot tolerate, other antihypertensive agents. It is prudent, if labetalol hydrochloride tablets are used, to use the smallest effective dose, so that inhibition of endogenous or exogenous beta-agonists is minimized.
Pheochromocytoma
Labetalol hydrochloride has been shown to be effective in lowering blood pressure and relieving symptoms in patients with pheochromocytoma. However, paradoxical hypertensive responses have been reported in a few patients with this tumor; therefore, use caution when administering labetalol hydrochloride to patients with pheochromocytoma.
Diabetes Mellitus and Hypoglycemia
Beta-adrenergic blockade may prevent the appearance of premonitory signs and symptoms (e.g. tachycardia) of acute hypoglycemia. This is especially important with labile diabetics. Beta-blockade also reduces the release of insulin in response to hyperglycemia; it may therefore be necessary to adjust the dose of antidiabetic drugs.
Major Surgery
Do not routinely withdraw chronic beta-blocker therapy prior to surgery. The effect of labetalol hydrochloride's alpha-adrenergic activity has not been evaluated in this setting.
A synergism between labetalol hydrochloride and halothane anesthesia has been shown (see PRECAUTIONS, Drug Interactions).
-
PRECAUTIONS
General
Impaired Hepatic Function
Labetalol hydrochloride tablets should be used with caution in patients with impaired hepatic function since metabolism of the drug may be diminished.Intraoperative Floppy Iris Syndrome (IFIS)
Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients treated with alpha-1 blockers (labetalol is an alpha/beta blocker). This variant of small pupil syndrome is characterized by the combinations of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. The patient’s ophthalmologist should be prepared for possible modifications to the surgical technique, such as the utilization of iris hooks, iris dilator rings, or viscoelastic substances. There does not appear to be a benefit of stopping alpha-1 blocker therapy prior to cataract surgery.Jaundice or Hepatic Dysfunction (see WARNINGS).
INFORMATION FOR PATIENTS
As with all drugs with beta-blocking activity, certain advice to patients being treated with labetalol hydrochloride is warranted. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects. While no incident of the abrupt withdrawal phenomenon (exacerbation of angina pectoris) has been reported with labetalol hydrochloride, dosing with labetalol hydrochloride tablets should not be interrupted or discontinued without a physician's advice. Patients being treated with labetalol hydrochloride tablets should consult a physician at any signs or symptoms of impending cardiac failure or hepatic dysfunction (see WARNINGS). Also, transient scalp tingling may occur, usually when treatment with labetalol hydrochloride tablets is initiated (see ADVERSE REACTIONS).
Laboratory Tests
As with any new drug given over prolonged periods, laboratory parameters should be observed over regular intervals. In patients with concomitant illnesses, such as impaired renal function, appropriate tests should be done to monitor these conditions.DRUG INTERACTIONS
In one survey, 2.3% of patients taking labetalol hydrochloride in combination with tricyclic antidepressants experienced tremor as compared to 0.7% reported to occur with labetalol hydrochloride alone. The contribution of each of the treatments to this adverse reaction is unknown but the possibility of a drug interaction cannot be excluded.
Drugs possessing beta-blocking properties can blunt the bronchodilator effect of beta-receptor agonist drugs in patients with bronchospasm; therefore, doses greater than the normal anti-asthmatic dose of beta-agonist bronchodilator drugs may be required.
Cimetidine has been shown to increase the bioavailability of labetalol hydrochloride. Since this could be explained either by enhanced absorption or by an alteration of hepatic metabolism of labetalol hydrochloride, special care should be used in establishing the dose required for blood pressure control in such patients.
Synergism has been shown between halothane anesthesia and intravenously administered labetalol hydrochloride. During controlled hypotensive anesthesia using labetalol hydrochloride in association with halothane, high concentrations (3% or above) of halothane should not be used because the degree of hypotension will be increased and because of the possibility of a large reduction in cardiac output and an increase in central venous pressure. The anesthesiologist should be informed when a patient is receiving labetalol hydrochloride.
Labetalol hydrochloride blunts the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effect. If labetalol hydrochloride is used with nitroglycerin in patients with angina pectoris, additional antihypertensive effects may occur.
Care should be taken if labetalol is used concomitantly with calcium antagonists of the verapamil type.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Risk of Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.Drug/Laboratory Test Interactions
The presence of labetalol metabolites in the urine may result in falsely elevated levels of urinary catecholamines, metanephrine, normetanephrine and vanillylmandelic acid (VMA) when measured by fluorimetric or photometric methods. In screening patients suspected of having a pheochromocytoma and being treated with labetalol hydrochloride, a specific method, such as a high performance liquid chromatographic assay with solid phase extraction (e.g. J. Chromatogr 385:241, 1987) should be employed in determining levels of catecholamines.
Labetalol hydrochloride has also been reported to produce a false-positive test for amphetamine when screening urine for the presence of drugs using the commercially available assay methods TOXI-Lab ® A (thin-layer chromatographic assay) and EMIT-d.a.u. ®(radioenzymatic assay). When patients being treated with labetalol have a positive urine test for amphetamine using these techniques, confirmation should be made by using more specific methods, such as a gas chromatographic-mass spectrometer technique.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term oral dosing studies with labetalol hydrochloride for 18 months in mice and for 2 years in rats showed no evidence of carcinogenesis. Studies with labetalol hydrochloride using dominant lethal assays in rats and mice and exposing microorganisms according to modified Ames tests, showed no evidence of mutagenesis.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Teratogenic studies were performed with labetalol in rats and rabbits at oral doses up to approximately six and four times the maximum recommended human dose (MRHD), respectively. No reproducible evidence of fetal malformations was observed. Increased fetal resorptions were seen in both species at doses approximating the MRHD. A teratology study performed with labetalol in rabbits at intravenous doses up to 1.7 times the MRHD revealed no evidence of drug-related harm to the fetus. There are no adequate and well-controlled studies in pregnant women. Labetalol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Nonteratogenic Effects
Hypotension, bradycardia, hypoglycemia and respiratory depression have been reported in infants of mothers who were treated with labetalol hydrochloride for hypertension during pregnancy. Oral administration of labetalol to rats during late gestation through weaning at doses of two to four times the MRHD caused a decrease in neonatal survival.Labor and Delivery
Labetalol hydrochloride given to pregnant women with hypertension did not appear to affect the usual course of labor and delivery.
Nursing Mothers
Small amounts of labetalol (approximately 0.004% of the maternal dose) are excreted in human milk. Caution should be exercised when labetalol hydrochloride tablets are administered to a nursing woman.
Elderly Patients
As in the general population, some elderly patients (60 years of age and older) have experienced orthostatic hypotension, dizziness or lightheadedness during treatment with labetalol. Because elderly patients are generally more likely than younger patients to experience orthostatic symptoms, they should be cautioned about the possibility of such side effects during treatment with labetalol.
-
ADVERSE REACTIONS
Most adverse effects are mild and transient and occur early in the course of treatment. In controlled clinical trials of 3 to 4 months duration, discontinuation of labetalol hydrochloride tablets due to one or more adverse effects was required in 7% of all patients. In these same trials, other agents with solely beta-blocking activity used in the control groups led to discontinuation in 8% to 10% of patients and acentrally acting alpha-agonist led to discontinuation in 30% of patients.
The incidence rates of adverse reactions listed in the following table were derived from multicenter controlled clinical trials, comparing labetalol hydrochloride, placebo, metoprolol and propranolol, over treatment periods of 3 and 4 months. Where the frequency of adverse effects for labetalol hydrochloride and placebo is similar, causal relationship is uncertain. The rates are based on adverse reactions considered probably drug related by the investigator. If all reports are considered, the rates are somewhat higher (e.g. dizziness 20%, nausea 14%, fatigue 11%), but the overall conclusions are unchanged.
Labetalol
Hydrochloride
(N=227) %Placebo
(N=98) %Propranolol
(N=84) %Metoprolol
(N=49) %Body as a whole
Fatigue
5
0
12
12
Asthenia
1
1
1
0
Headache
2
1
1
2
Gastrointestinal
Nausea
6
1
1
2
Vomiting
Less than 1
0
0
0
Dyspepsia
3
1
1
0
Abdominal pain
0
0
1
2
Diarrhea
Less than 1
0
2
0
Taste distortion
1
0
0
0
Central and Peripheral Nervous Systems
Dizziness
11
3
4
4
Paresthesias
Less than 1
0
0
0
Drowsiness
Less than 1
2
2
2
Autonomic Nervous System
Nasal stuffiness
3
0
0
0
Ejaculation failure
2
0
0
0
Impotence
1
0
1
3
Increased sweating
Less than 1
0
0
0
Cardiovascular
Edema
1
0
0
0
Postural hypotension
1
0
0
0
Bradycardia
0
0
5
12
Respiratory
Dyspnea
2
0
1
2
Skin
Rash
1
0
0
0
Special Senses
Vision abnormality
1
0
0
0
Vertigo
2
1
0
0
The adverse effects were reported spontaneously and are representative of the incidence of adverse effects that may be observed in a properly selected hypertensive patient population, i.e., a group excluding patients with bronchospastic disease, overt congestive heart failure, or other contraindications to beta-blocker therapy.
Clinical trials also included studies utilizing daily doses up to 2,400 mg in more severely hypertensive patients. Certain of the side effects increased with increasing dose as shown in the table below that depicts the entire U.S. therapeutic trials data base for adverse reactions that are clearly or possibly dose related.
Labetalol Hydrochloride Daily Dose (mg)
200
300
400
600
800
900
1200
1600
2400
Number of Patients
522
181
606
608
503
117
411
242
175
Dizziness (%)
2
3
3
3
5
1
9
13
16
Fatigue
2
1
4
4
5
3
7
6
10
Nausea
Less than 1
0
1
2
4
0
7
11
19
Vomiting
0
0
Less than 1
Less than 1
Less than 1
0
1
2
3
Dyspepsia
1
0
2
1
1
0
2
2
4
Paresthesia
2
0
2
2
1
1
2
5
5
Nasal Stuffiness
1
1
2
2
2
2
4
5
6
Ejaculation Failure
0
2
1
2
3
0
4
3
5
Impotence
1
1
1
1
2
4
3
4
3
Edema
1
0
1
1
1
0
1
2
2
In addition, a number of other less common adverse events have been reported:
Body as a Whole: Fever.
Cardiovascular:Hypotension, and rarely, syncope, bradycardia, heart block.
Central and Peripheral Nervous Systems: Paresthesia, most frequently described as scalp tingling. In most cases, it was mild and transient and usually occurred at the beginning of treatment.
Collagen Disorders: Systemic lupus erythematosus; positive antinuclear factor (ANF).
Eyes: Dry eyes.
Immunological System: Anti mitochondrial antibodies.
Liver and Biliary System: Hepatic necrosis; hepatitis; cholestatic jaundice; elevated liver function tests.
Musculoskeletal System: Muscle cramps; toxic myopathy.
Respiratory System: Bronchospasm.
Skin and Appendages:Rashes of various types, such as generalized maculopapular, lichenoid, urticarial, bullous lichen planus, psoriaform, and facial erythema; Peyronie's disease, reversible alopecia.
Urinary System: Difficulty in micturition, including acute urinary bladder retention.
Hypersensitivity: Rare reports of hypersensitivity (e.g. rash, urticaria, pruritus, angioedema, dyspnea) and anaphylactoid reactions.
Following approval for marketing in the United Kingdom, a monitored release survey involving approximately 6,800 patients was conducted for further safety and efficacy evaluation of this product. Results of this survey indicate that the type, severity and incidence of adverse effects were comparable to those cited above.
Potential Adverse Effects
In addition, other adverse effects not listed above have been reported with other beta-adrenergic blocking agents.
Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium and decreased performance on psychometrics.
Cardiovascular: Intensification of A-V block (see CONTRAINDICATIONS).
Allergic: Fever combined with aching and sore throat; laryngospasm; respiratory distress.
Hematologic : Agranulocytosis; thrombocytopenic or non thrombocytopenic purpura.
Gastrointestinal: Mesenteric artery thrombosis; ischemic colitis.
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with labetalol hydrochloride.
Clinical Laboratory Tests
There have been reversible increases of serum transaminases in 4% of patients treated with labetalol hydrochloride and tested, and more rarely, reversible increases in blood urea. -
OVERDOSAGE
Overdosage with labetalol hydrochloride causes excessive hypotension that is posture sensitive and sometimes excessive bradycardia. Patients should be placed supine and their legs raised, if necessary, to improve the blood supply to the brain. If overdosage with labetalol hydrochloride follows oral ingestion, gastric lavage or pharmacologically induced emesis (using syrup of ipecac) may be useful for removal of the drug shortly after ingestion. The following additional measures should be employed if necessary:
Excessive bradycardia- administer atropine or epinephrine.
Cardiac failure- administer a digitalis glycoside and a diuretic. Dopamine or dobutamine may also be useful.
Hypotension- administer vasopressors, e.g., norepinephrine. There is pharmacologic evidence that norepinephrine may be the drug of choice.
Bronchospasm- administer epinephrine and/or an aerosolized beta 2-agonist.
Seizures– administer diazepam.
In severe beta-blocker overdose resulting in hypotension and/or bradycardia, glucagon has been shown to be effective when administered in large doses (5 mg to 10 mg rapidly over 30 seconds, followed by continuous infusion of 5 mg per hour that can be reduced as the patient improves).
Neither hemodialysis nor peritoneal dialysis removes a significant amount of labetalol hydrochloride from the general circulation (<1%).
The oral LD 50 value of labetalol hydrochloride in the mouse is approximately 600 mg/kg and in the rat is greater > 2 g/kg. The intravenous LD 50 in these species is 50 mg/kg to 60 mg/kg.
-
DOSAGE AND ADMINISTRATION
DOSAGE MUST BE INDIVIDUALIZED. The recommended initial dosage is 100 mg twicedaily whether used alone or added to a diuretic regimen. After 2 or 3 days, using standing blood pressure as an indicator, dosage may be titrated in increments of 100 mg b.i.d. every 2 or 3 days. The usual maintenance dosage of labetalol hydrochloride tablets is between 200 mg and 400 mg twice daily.
Since the full antihypertensive effect of labetalol hydrochloride tablets is usually seen within the first 1 to 3 hours of the initial dose or dose increment, the assurance of a lack of an exaggerated hypotensive response can be clinically established in the office setting. The antihypertensive effects of continued dosing can be measured at subsequent visits, approximately 12 hours after a dose, to determine whether further titration is necessary.
Patients with severe hypertension may require from 1200 mg to 2400 mg per day, with or without thiazide diuretics. Should side effects (principally nausea or dizziness) occur with these doses administered b.i.d. (twice daily), the same total daily dose administered t.i.d. (three times daily) may improve tolerability and facilitate further titration. Titration increments should not exceed 200 mg b.i.d. (twice daily).
When a diuretic is added, an additive antihypertensive effect can be expected. In some cases this may necessitate a labetalol hydrochloride tablet dosage adjustment. As with most antihypertensive drugs, optimal dosages of labetalol hydrochloride tablets are usually lower in patients also receiving a diuretic. When transferring a patient from other antihypertensive drugs, labetalol hydrochloride tablets should be introduced as recommended and the dosage of the existing therapy progressively decreased.
Elderly Patients
As in the general patient population, labetalol therapy may be initiated at 100 mg twice daily and titrated upwards in increments of 100 mg b.i.d. as required for control of blood pressure. Since some elderly patients eliminate labetalol more slowly, however, adequate control of blood pressure may be achieved at a lower maintenance dosage compared to the general population. The majority of elderly patients will require between 100 mg and 200 mg b.i.d. -
HOW SUPPLIED
Labetalol Hydrochloride Tablets, USP for oral administration are available as:
Labetalol Hydrochloride Tablets USP, 100 mg are yellow colored, round, biconvex, film-coated tablets debossed with "7" and "98" on either side of score line on one side and plain on other side and are supplied as:
Unit dose packages of 100 (10 x 10) NDC 60687-439-01Labetalol Hydrochloride Tablets USP, 200 mg are white to off white colored, round, biconvex, film-coated tablets debossed with "7" and "99" on either side of score line on one side and plain on other side and are supplied as:
Unit dose packages of 100 (10 x 10) NDC 60687-450-01Labetalol Hydrochloride Tablets USP, 300 mg are light green colored, round, biconvex, beveled edge, film-coated tablets debossed with "800" on one side and plain on other side and are supplied as:
Unit dose packages of 100 (10 x 10) NDC 60687-461-01Labetalol Hydrochloride Tablets USP should be stored at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This product's package insert may have been updated. For current package insert, call American Health Packaging at 1-800-707-4621.
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see HOW SUPPLIED section) contain drug product from BluePoint Laboratories as follows:
(100 mg / 100UD) NDC 60687-439-01 packaged from NDC 68001-381
(200 mg / 100UD) NDC 60687-450-01 packaged from NDC 68001-382
(300 mg / 100UD) NDC 60687-461-01 packaged from NDC 68001-383Distributed by:
American Health Packaging
Columbus, OH 432178443901/0823
-
Package/Label Display Panel – Carton – 100 mg

NDC 60687- 439-01
Labetalol
Hydrochloride
Tablets, USP100 mg
100 Tablets (10 x 10) Rx Only
Each Film-Coated Tablet Contains:
Labetalol Hydrochloride, USP................................................100 mgUsual Dosage: See package insert for full prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 68001-381, BluePoint Laboratories.Distributed by:
American Health Packaging
Columbus, Ohio 43217743901
0443901/0222 - Package/Label Display Panel – Blister – 100 mg
-
Package/Label Display Panel – Carton – 200 mg

NDC 60687- 450-01
Labetalol
Hydrochloride
Tablets, USP200 mg
100 Tablets (10 x 10) Rx Only
Each Film-Coated Tablet Contains:
Labetalol Hydrochloride, USP................................................200 mgUsual Dosage: See package insert for full prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 68001-382, BluePoint Laboratories.Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217745001
0445001/0119 - Package/Label Display Panel – Blister – 200 mg
-
Package/Label Display Panel – Carton – 300 mg

NDC 60687- 461-01
Labetalol
Hydrochloride
Tablets, USP300 mg
100 Tablets (10 x 10) Rx Only
Each Film-Coated Tablet Contains:
Labetalol Hydrochloride, USP....................................300 mgUsual Dosage: See package insert for full prescribing
information.Store at 20° to 25°C (68° to 77°F); excursions permitted
between 15° to 30°C (59° to 86°F) [see USP Controlled
Room Temperature].Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or
broken.The drug product contained in this package is from
NDC # 68001-383, BluePoint Laboratories.Distributed by:
American Health Packaging
Columbus, Ohio 43217746101
0446101/1022 - Package/Label Display Panel – Blister – 300 mg
-
INGREDIENTS AND APPEARANCE
LABETALOL HYDROCHLORIDE
labetalol hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60687-439(NDC:68001-381) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LABETALOL HYDROCHLORIDE (UNII: 1GEV3BAW9J) (LABETALOL - UNII:R5H8897N95) LABETALOL HYDROCHLORIDE 100 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color yellow (YELLOW) Score 2 pieces Shape ROUND (BICONVEX) Size 8mm Flavor Imprint Code 7;98 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60687-439-01 100 in 1 BOX, UNIT-DOSE 07/16/2019 1 NDC:60687-439-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 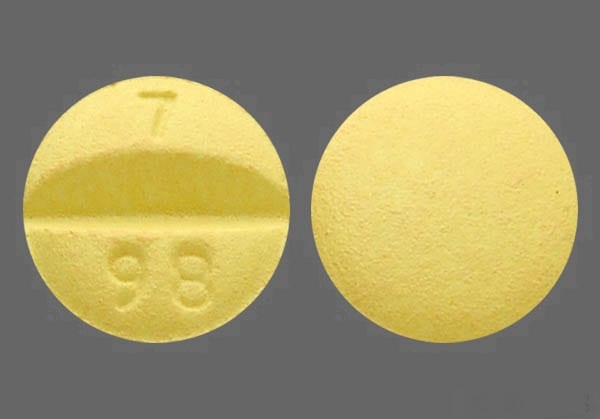
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207743 07/16/2019 LABETALOL HYDROCHLORIDE
labetalol hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60687-450(NDC:68001-382) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LABETALOL HYDROCHLORIDE (UNII: 1GEV3BAW9J) (LABETALOL - UNII:R5H8897N95) LABETALOL HYDROCHLORIDE 200 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (OFF-WHITE) Score 2 pieces Shape ROUND (BICONVEX) Size 11mm Flavor Imprint Code 7;99 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60687-450-01 100 in 1 BOX, UNIT-DOSE 07/16/2019 1 NDC:60687-450-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207743 07/16/2019 LABETALOL HYDROCHLORIDE
labetalol hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60687-461(NDC:68001-383) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LABETALOL HYDROCHLORIDE (UNII: 1GEV3BAW9J) (LABETALOL - UNII:R5H8897N95) LABETALOL HYDROCHLORIDE 300 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color green (LIGHT GREEN) Score no score Shape ROUND (BICONVEX) Size 11mm Flavor Imprint Code 800 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60687-461-01 100 in 1 BOX, UNIT-DOSE 07/16/2019 1 NDC:60687-461-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 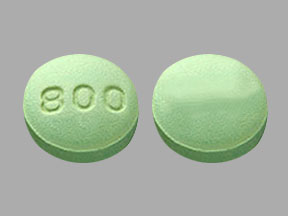
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207743 07/16/2019 Labeler - American Health Packaging (929561009) Establishment Name Address ID/FEI Business Operations American Health Packaging 929561009 repack(60687-439, 60687-450, 60687-461)









