Label: ACYCLOVIR tablet
- NDC Code(s): 60505-5306-1, 60505-5306-3, 60505-5306-5, 60505-5306-8, view more
- Packager: Apotex Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
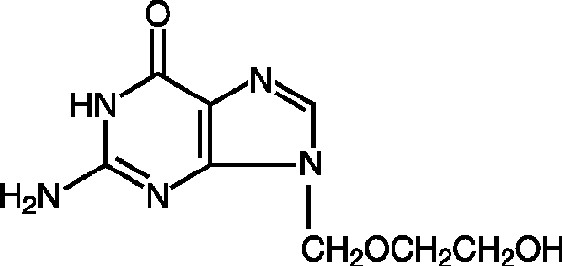
DESCRIPTIONAcyclovir is a synthetic nucleoside analogue active against herpes viruses. Acyclovir tablets are a formulation for oral administration. Each 800 mg tablet of acyclovir contains 800 mg of ...
-
VIROLOGYMechanism of Antiviral Action - Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and ...
-
CLINICAL PHARMACOLOGYPharmacokinetics - The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster ...
-
INDICATIONS AND USAGEHerpes Zoster Infections - Acyclovir tablets are indicated for the acute treatment of herpes zoster (shingles). Genital Herpes - Acyclovir tablets are indicated for the treatment of initial ...
-
CONTRAINDICATIONSAcyclovir is contraindicated for patients who develop hypersensitivity to acyclovir or valacyclovir.
-
WARNINGSAcyclovir tablets are intended for oral ingestion only. Renal failure, in some cases resulting in death, has been observed with acyclovir therapy (see ADVERSE REACTIONS: Observed During Clinical ...
-
PRECAUTIONSDosage adjustment is recommended when administering acyclovir to patients with renal impairment (see DOSAGE AND ADMINISTRATION). Caution should also be exercised when administering acyclovir to ...
-
ADVERSE REACTIONSHerpes Simplex - Short-Term Administration - The most frequent - adverse events reported during clinical trials of treatment of genital herpes - with acyclovir 200 mg administered orally ...
-
OVERDOSAGEOverdoses involving ingestion of up to 100 capsules (20 g) have been reported. Adverse events that have been reported in association with overdosage include agitation, coma, seizures, and ...
-
DOSAGE AND ADMINISTRATIONAcute Treatment of Herpes Zoster - 800 mg every 4 hours orally, 5 times daily - for 7 to 10 days. Genital Herpes - Treatment of Initial Genital Herpes - 200 mg every - 4 hours, 5 times daily ...
-
HOW SUPPLIEDAcyclovir Tablets, USP 400 mg are available for oral administration as white to off-white, oval, unscored tablets imprinted "Apotex Logo 5306" on one side and plain on the other side. They are ...
-
PRINCIPAL DISPLAY PANEL - 400 mgRepresentative sample of the labeling (see the HOW SUPPLIED section for complete listing): PRINCIPAL DISPLAY PANEL - 400 mg BOTTLE LABEL - APOTEX CORP. NDC 60505-5306-1 - ACYCLOVIR TABLETS, USP ...
-
PRINCIPAL DISPLAY PANEL - 800 mgRepresentative sample of the labeling (see the HOW SUPPLIED section for complete listing): PRINCIPAL DISPLAY PANEL - 800 mg BOTTLE LABEL - APOTEX CORP. NDC 60505-5307-1 - ACYCLOVIR TABLETS, USP ...
-
INGREDIENTS AND APPEARANCEProduct Information