Label: TRAZODONE HYDROCHLORIDE tablet
- NDC Code(s): 60505-2653-0, 60505-2653-1, 60505-2653-5, 60505-2653-7, view more
- Packager: Apotex Corp
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 20, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRAZODONE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for TRAZODONE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL THOUGHTS and BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. Trazodone hydrochloride tablets are not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
Close -
1 INDICATIONS AND USAGE
Trazodone hydrochloride tablets are indicated for the treatment of major depressive disorder (MDD) in adults.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dose Selection - An initial dose of 150 mg/day in divided doses is suggested. The dosage should be initiated at a low-dose and increased gradually, noting the clinical response and any ...
-
3 DOSAGE FORMS AND STRENGTHS
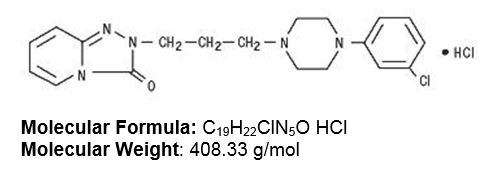
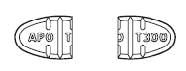
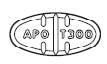
Trazodone hydrochloride tablets, USP are available in the following strengths: 50 mg : white, round, biconvex, scored tablets. Engraved “APO” bisect “T50” on one side, other side plain. 100 mg ...
-
4 CONTRAINDICATIONS
Trazodone hydrochloride tablets are contraindicated in: Patients taking, or within 14 days of stopping, monoamine oxidase inhibitors (MAOIs), including MAOIs such as linezolid or intravenous ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Pediatric and Young Adult Patients - In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Suicidal Thoughts and Behavior in Children, Adolescents and Young Adults [see Boxed Warning and Warnings and ...
-
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions With Trazodone Hydrochloride Tablets - Table 3: Clinically Important Drug Interactions with Trazodone Hydrochloride Tablets - Monoamine ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare providers ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - Trazodone hydrochloride tablets are not a controlled substance. 9.2 Abuse - Although trazodone hydrochloride has not been systematically studied in preclinical or ...
-
10 OVERDOSAGE
Death from overdose has occurred in patients ingesting trazodone and other CNS depressant drugs concurrently (alcohol; alcohol and chloral hydrate and diazepam; amobarbital; chlordiazepoxide; or ...
-
11 DESCRIPTIONTrazodone hydrochloride is an antidepressant chemically unrelated to tricyclic, tetracyclic, or other known antidepressant agents. Trazodone hydrochloride is a triazolopyridine derivative ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The mechanism of trazodone’s antidepressant action is not fully understood, but is thought to be related to its enhancement of serotonergic activity in the CNS ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No drug- or dose-related occurrence of carcinogenesis was evident in rats receiving trazodone in daily oral doses up ...
-
14 CLINICAL STUDIES
The efficacy and safety of trazodone hydrochloride were established from inpatient and outpatient trials of the trazodone immediate release formulation in the treatment of major depressive ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Trazodone hydrochloride tablets, USP 50 mg are available for oral administration as white, round, biconvex, tablets. Engraved “APO” bisect “T50” on one side, other side plain. Bottles of 100 (NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Suicidal Thoughts and Behaviors - Advise patients and caregivers to look for the emergence of suicidality ...
-
MEDICATION GUIDE
Trazodone hydrochloride tablets, USP, for oral use - (traz' oh done hye'' droe klor' ide) Medication Guide available at https://www.apotex.com/products/us/mg.asp - What is the most important ...
-
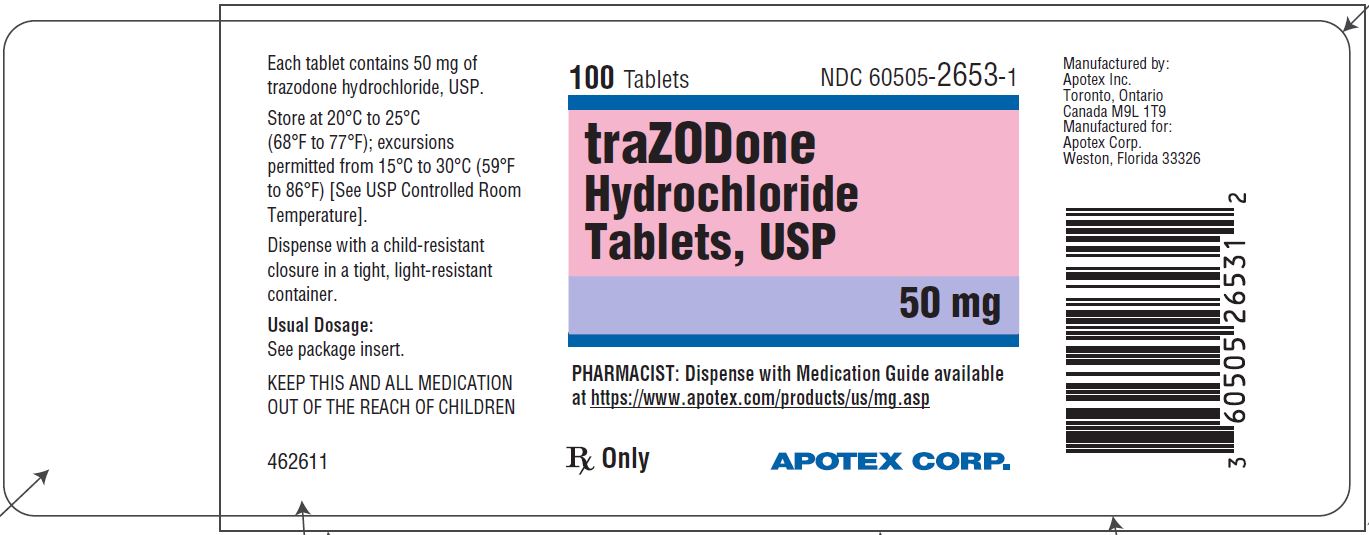
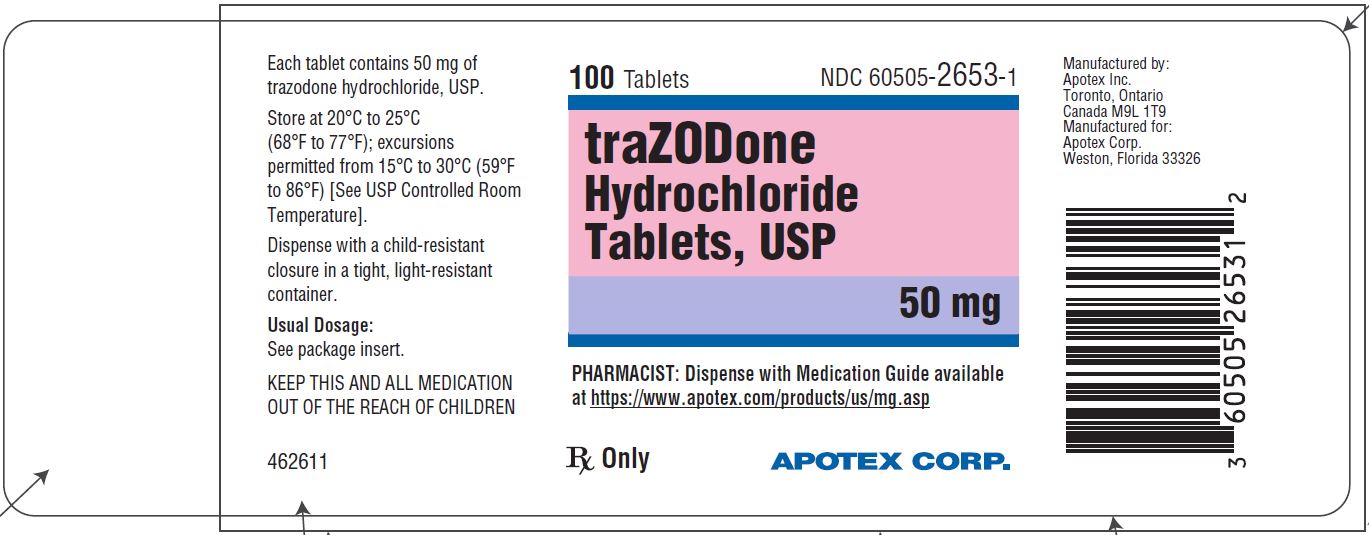
PRINCIPAL DISPLAY PANEL - 50 mg BOTTLE LABEL - APOTEX CORP. NDC 60505-2653-1 - TRAZODONE HYDROCHLORIDE TABLETS USP - 50 mg - Rx only - 100 Tablets
-
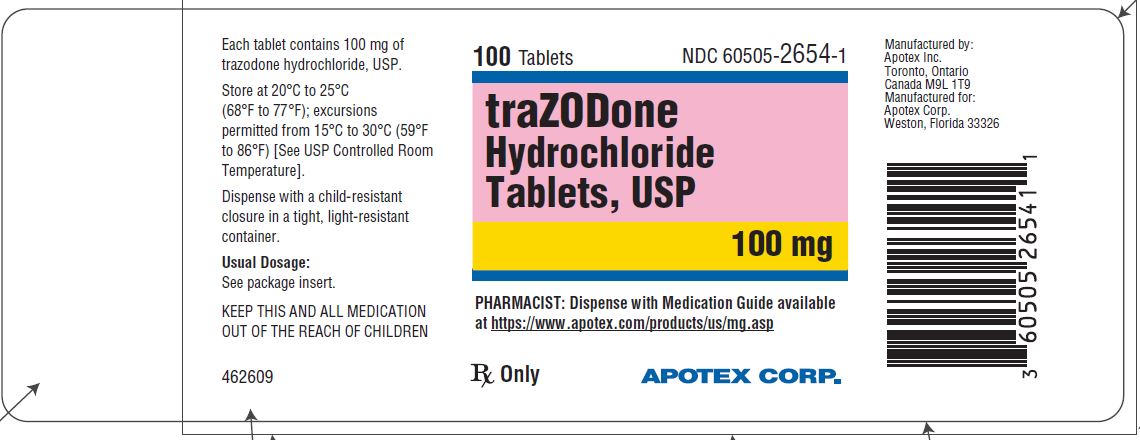
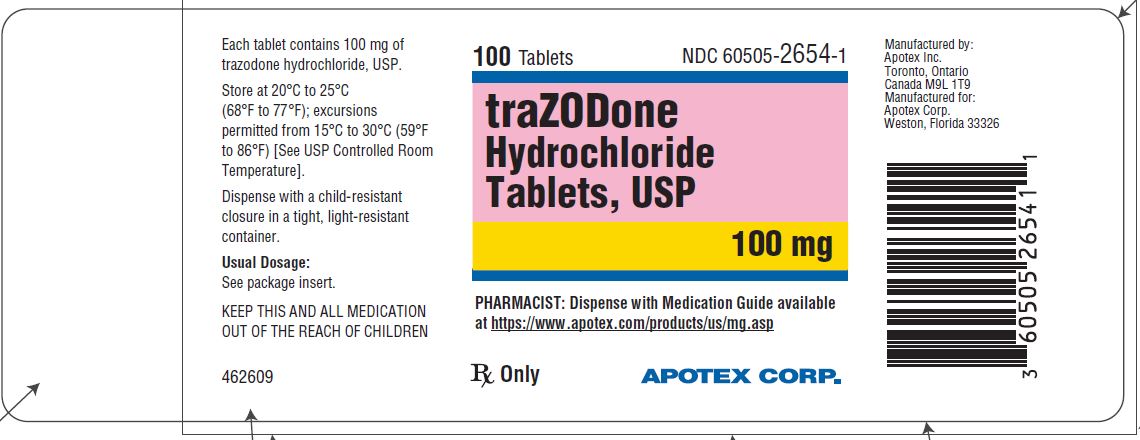
PRINCIPAL DISPLAY PANEL - 100 mg BOTTLE LABEL - APOTEX CORP. NDC 60505-2654-1 - TRAZODONE HYDROCHLORIDE TABLETS USP - 100 mg - Rx only - 100 Tablets
-
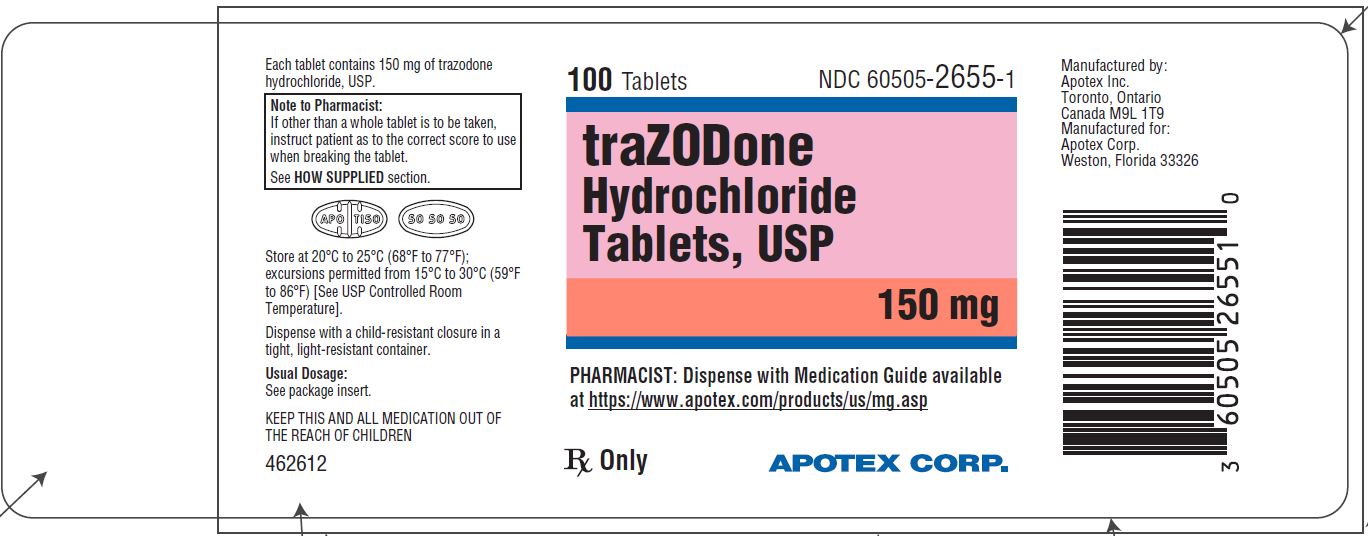
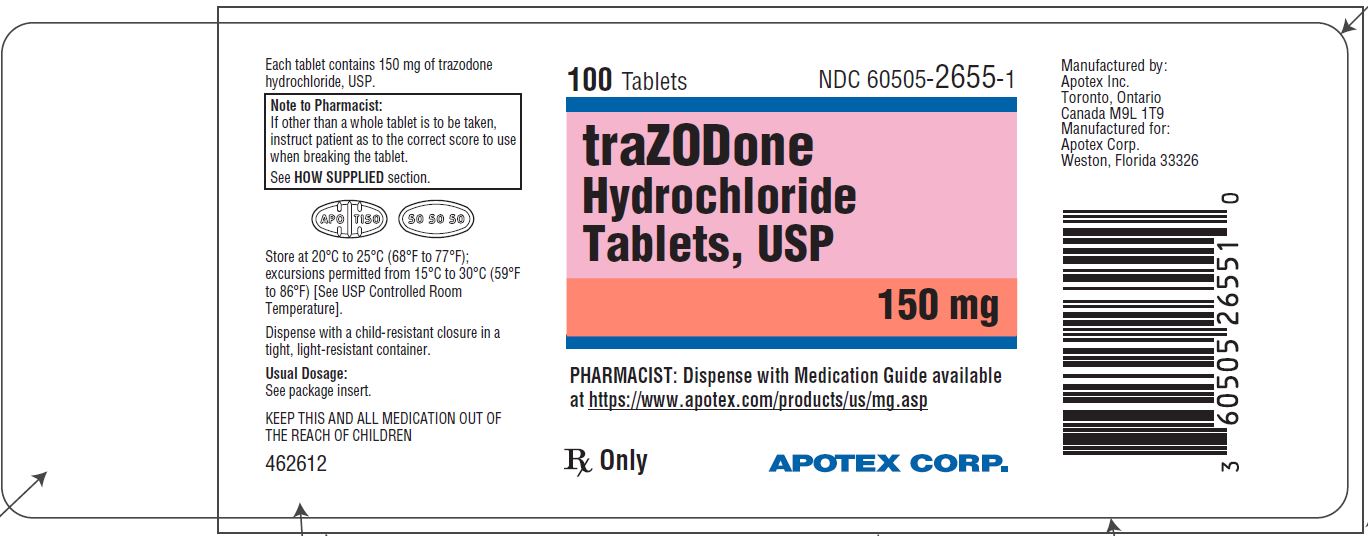
PRINCIPAL DISPLAY PANEL - 150 mg BOTTLE LABEL - APOTEX CORP. NDC 60505-2655-1 - TRAZODONE HYDROCHLORIDE TABLETS USP - 150 mg - Rx only - 100 Tablets
-
PRINCIPAL DISPLAY PANEL - 150 mg BOTTLE LABEL - APOTEX CORP. NDC 60505-4089-3 - TRAZODONE HYDROCHLORIDE TABLETS USP - 150 mg - Rx only - 12 Bottles x 30 Tablets per bottle
-
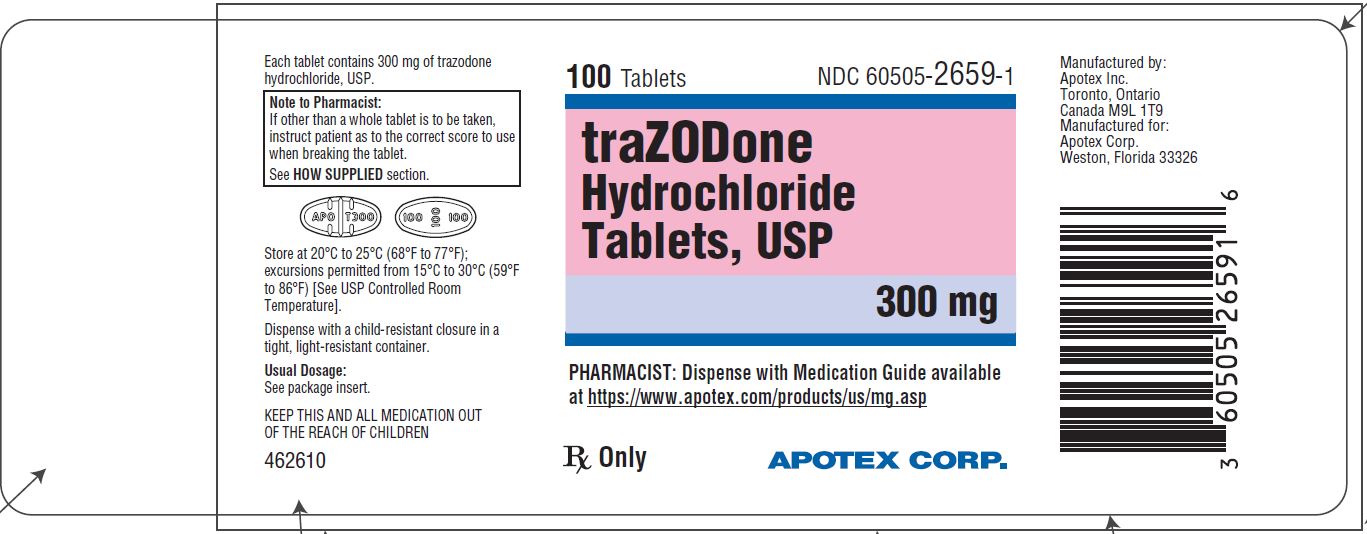
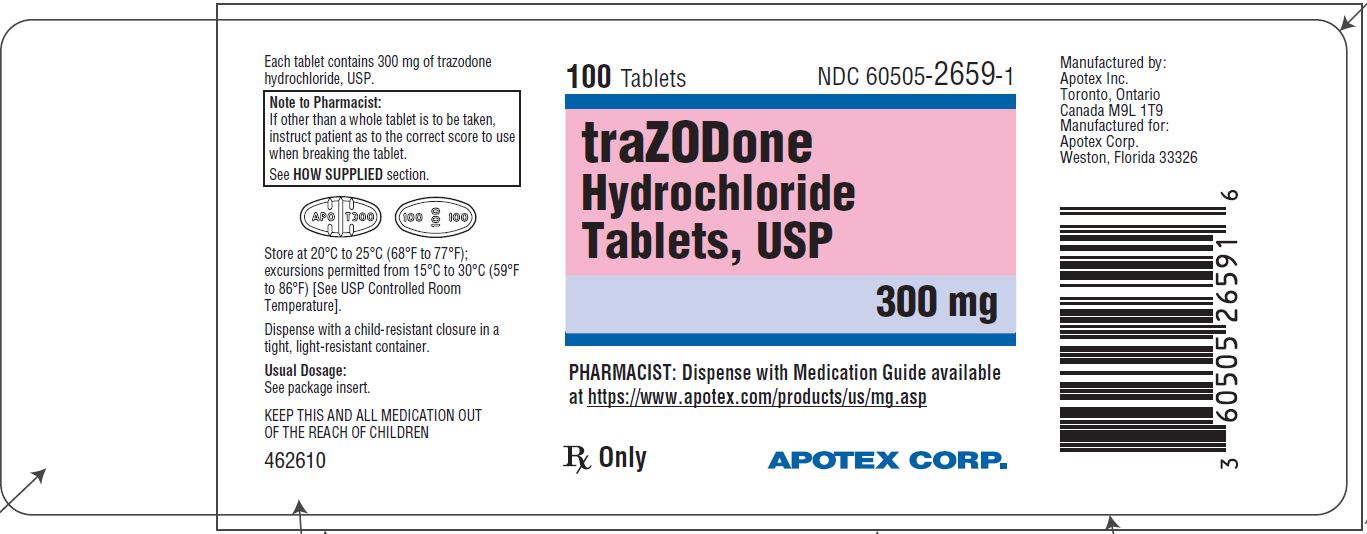
PRINCIPAL DISPLAY PANEL - 300 mg BOTTLE LABEL - APOTEX CORP. NDC 60505-2659-1 - TRAZODONE HYDROCHLORIDE TABLETS USP - 300 mg - Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information