Label: DESMOPRESSIN ACETATE spray
- NDC Code(s): 60505-0815-0
- Packager: Apotex Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DESMOPRESSIN NASAL SPRAY safely and effectively. See full prescribing information for DESMOPRESSIN NASAL SPRAY. DESMOPRESSIN ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDesmopressin nasal spray is indicated as antidiuretic replacement therapy in the management of central diabetes insipidus in adults and pediatric patients 4 years of age and older Limitations ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Administer desmopressin nasal spray by intranasal use only. Instruct patients about appropriate fluid restriction during desmopressin nasal spray ...
-
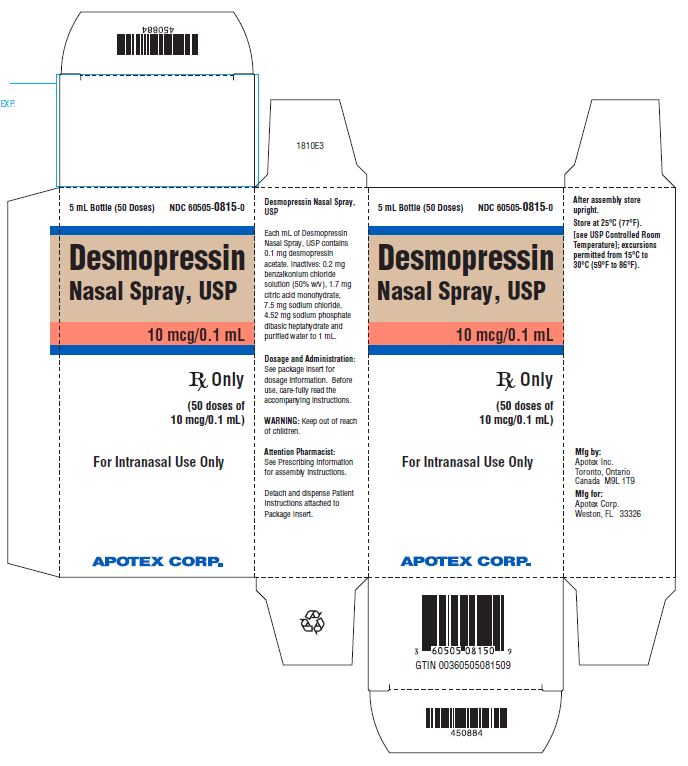
3 DOSAGE FORMS AND STRENGTHSDesmopressin nasal spray pump delivers 10 mcg (0.1 mL) of desmopressin acetate per spray. Desmopressin nasal spray is available as a 5 mL bottle with spray pump delivering 50 sprays.
-
4 CONTRAINDICATIONSDesmopressin nasal spray is contraindicated in patients with: Known hypersensitivity to desmopressin acetate or to any of the components of desmopressin nasal spray. Severe allergic reactions and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hyponatremia - Excessive fluid intake when urine output is limited by the antidiuretic effect of desmopressin may lead to water intoxication with hyponatremia. Cases of hyponatremia have ...
-
6 ADVERSE REACTIONSThe following serious reactions are described below and elsewhere in the labeling: Hyponatremia [see Warnings and Precautions (5.1)]. Altered Absorption in Patients with Changes in Nasal Mucosa ...
-
7 DRUG INTERACTIONS7.1 Other Drugs that may Increase Risk of Hyponatremia - The concomitant administration of desmopressin nasal spray with other drugs that may increase the risk of water intoxication with ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk summary Prolonged experience with desmopressin in pregnant women over several decades, based on the available published data and case reports, did not identify a drug ...
-
10 OVERDOSAGESigns of desmopressin acetate overdosage may include confusion, drowsiness, continuing headache, problems with passing urine, and rapid weight gain due to fluid retention [see Warnings and ...
-
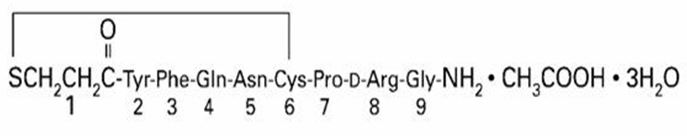
11 DESCRIPTIONDesmopressin nasal spray, USP is a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. The structural ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The antidiuretic effects of desmopressin are mediated by stimulation of vasopressin 2 (V2) receptors, thereby increasing water re-absorption in the kidney, and hence ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies with desmopressin acetate have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Desmopressin nasal spray, USP is available in a 5 mL bottle with a nasal spray pump dispenser with dust cover and patient instruction sheet delivering 50 sprays of 10 mcg ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use) Administration - Inform caregivers for pediatric patients that administration ...
-
Patient InformationDesmopressin Nasal Spray, USP 10 mcg per 0.1 mL - (des·mo·pres·sin) For Intranasal Use Only - What is desmopressin nasal spray? Desmopressin nasal spray is a prescription medicine called a ...
-
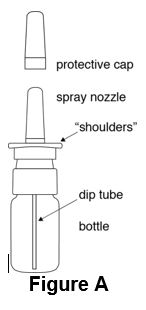
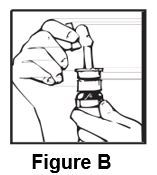
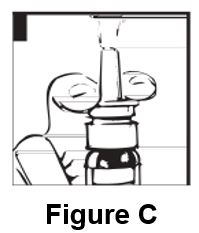
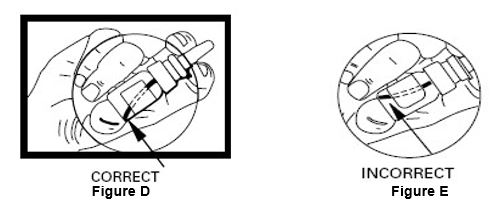
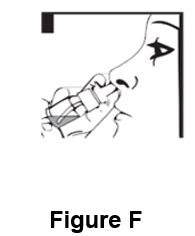
Instructions for UseDesmopressin Nasal Spray, USP 10 mcg per 0.1 mL - For Intranasal Use Only Read these instructions before using desmopressin nasal spray, and each time you get a refill. There may be new ...
-
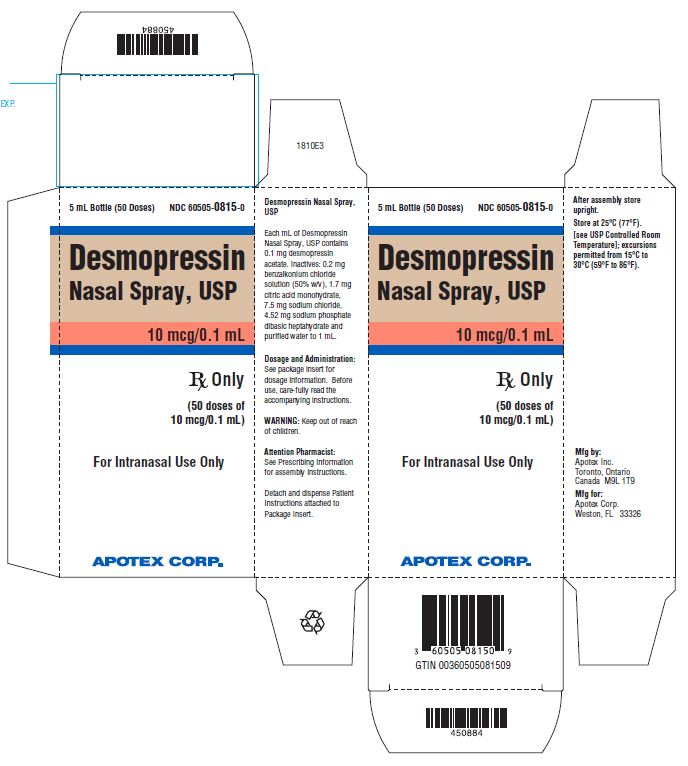
PRINCIPAL DISPLAY PANEL - 5 mL Carton LabelAPOTEX CORP. NDC 60505-0815-0 - Desmopressin Nasal Spray Solution USP, 0.01% Rx Only - 5 mL
-
PRINCIPAL DISPLAY PANEL - 5 mL Bottle LabelAPOTEX CORP. NDC 60505-0815-0 - Desmopressin Nasal Spray Solution USP, 0.01% Rx Only - 5 mL
-
INGREDIENTS AND APPEARANCEProduct Information