Label: MEGESTROL ACETATE suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 60432-126-08, 60432-126-16 - Packager: Morton Grove Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 10, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
DESCRIPTION
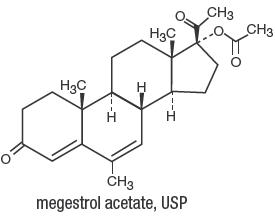
Megestrol Acetate Oral Suspension, USP contains megestrol acetate, a synthetic derivative of the steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid chemically ...
-
CLINICAL PHARMACOLOGYThe precise mechanism by which megestrol acetate produces effects in anorexia and cachexia is unknown at the present time. There are several analytical methods used to estimate megestrol acetate ...
-
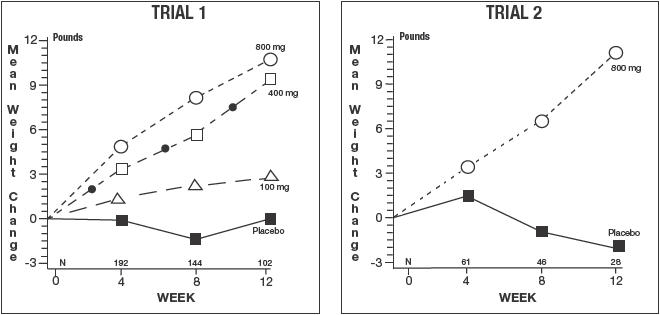
DESCRIPTION OF CLINICAL STUDIESThe clinical efficacy of Megestrol Acetate Oral Suspension, USP was assessed in two clinical trials. One was a multicenter, randomized, double-blind, placebo-controlled study comparing megestrol ...
-
INDICATIONS AND USAGEMegestrol Acetate Oral Suspension, USP is indicated for the treatment of anorexia, cachexia, or an unexplained, significant weight loss in patients with a diagnosis of acquired immunodeficiency ...
-
CONTRAINDICATIONSHistory of hypersensitivity to megestrol acetate or any component of the formulation. Known or suspected pregnancy.
-
WARNINGSMegestrol acetate may cause fetal harm when administered to a pregnant woman. For animal data on fetal effects, see PRECAUTIONS: Carcinogenesis, Mutagenesis, Impairment of Fertility: Impairment ...
-
PRECAUTIONSGeneral - Therapy with Megestrol Acetate Oral Suspension, USP for weight loss should only be instituted after treatable causes of weight loss are sought and addressed. These treatable causes ...
-
ADVERSE REACTIONSClinical Adverse Events - Adverse events which occurred in at least 5% of patients in any arm of the two clinical efficacy trials and the open trial are listed below by treatment group. All ...
-
OVERDOSAGENo serious unexpected side effects have resulted from studies involving Megestrol Acetate Oral Suspension, USP administered in dosages as high as 1200 mg/day. In post-marketing experience ...
-
DOSAGE AND ADMINISTRATIONThe recommended adult initial dosage of Megestrol Acetate Oral Suspension, USP is 800mg/day (20 mL/day). Shake container well before using. In clinical trials evaluating different dose schedules ...
-
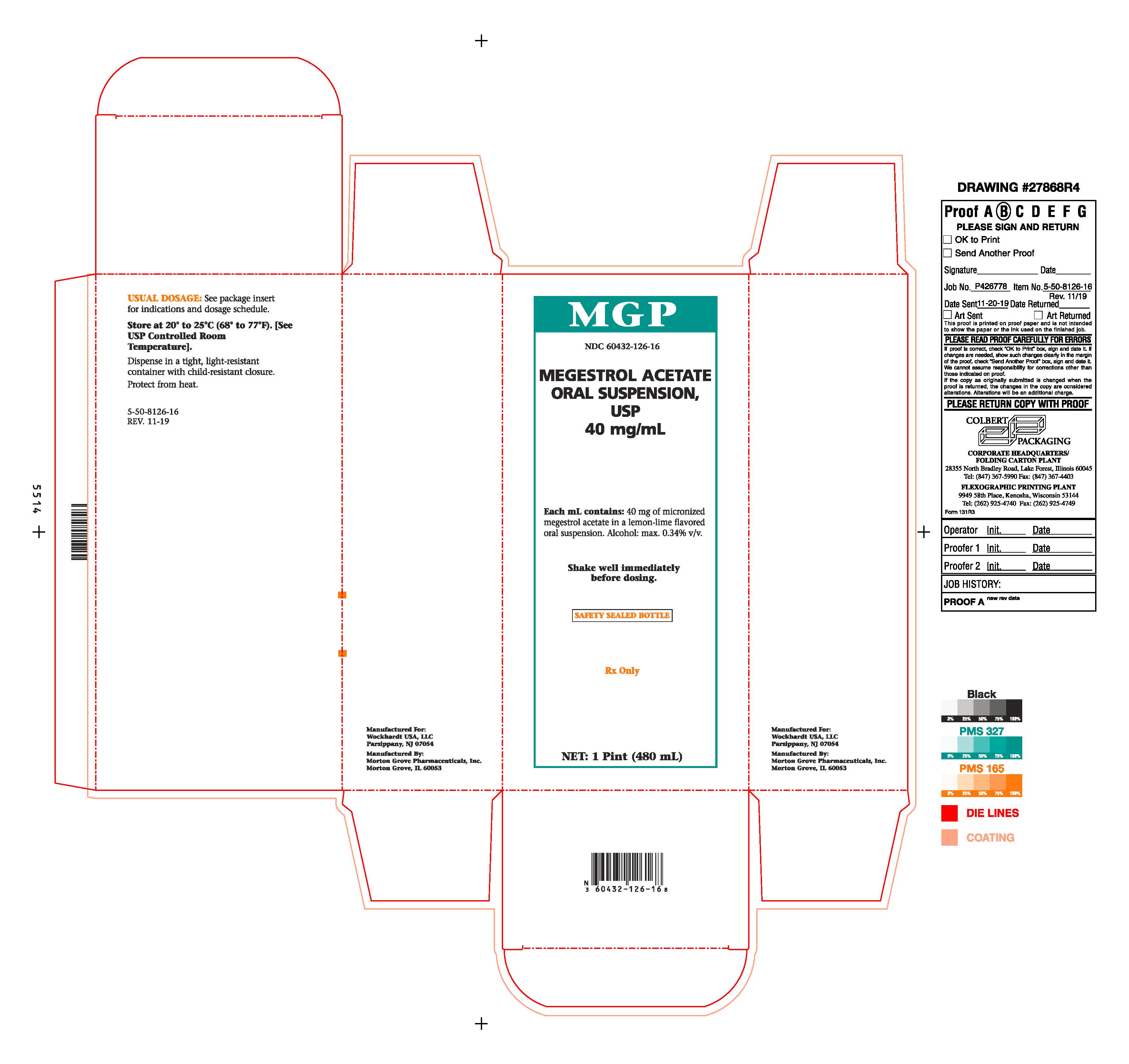
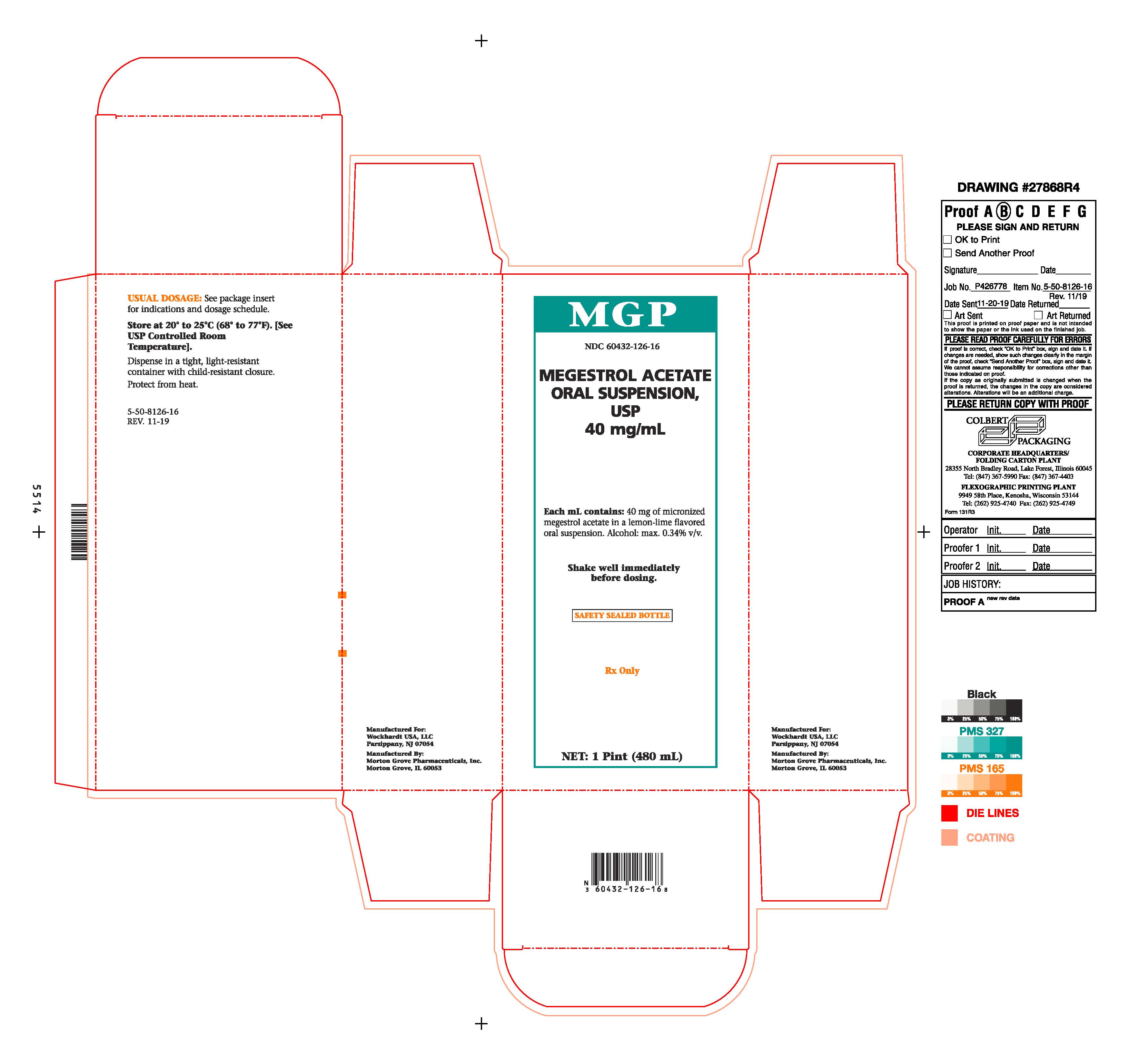
HOW SUPPLIEDMegestrol Acetate Oral Suspension, USP 40 mg/mL is available as a creamy-white, lemon-lime flavored oral suspension, for oral administration, containing 40 mg of micronized megestrol acetate per ...
-
STORAGEStore at 20 ° to 25 °C (68 ° to 77 °F). [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container with child-resistant closure. Protect from heat.
-
SPECIAL HANDLINGHealth Hazard Data - There is no threshold limit value established by OSHA, NIOSH, or ACGIH. Exposure or "overdose" at levels approaching recommended dosing levels could result in side effects ...
-
PRINCIPAL DISPLAY PANEL Bottle CartonMGP - NDC 60432-126-16 - MEGESTROL ACETATE - ORAL SUSPENSION, USP - 40 mg/mL - Each mL contains: 40 mg of micronized - megestrol acetate in a lemon-lime flavored - oral suspension. Alcohol: max ...
-
INGREDIENTS AND APPEARANCEProduct Information