Label: PILOCARPINE HYDROCHLORIDE solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 60219-1745-8, 60219-1746-8, 60219-1747-8 - Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 22, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PILOCARPINE HYDROCHLORIDE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for PILOCARPINE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPilocarpine hydrochloride ophthalmic solution is indicated for the: 1.1 Reduction of Elevated Intraocular Pressure - (IOP) in Patients with Open-Angle Glaucoma or Ocular Hypertension - 1.2 ...
-
2 DOSAGE AND ADMINISTRATION2.1 Reduction of Elevated - Intraocular Pressure (IOP) in Patients with Open-Angle Glaucoma or Ocular - Hypertension - One drop of pilocarpine hydrochloride ophthalmic solution 1%, 2% or 4% should be ...
-
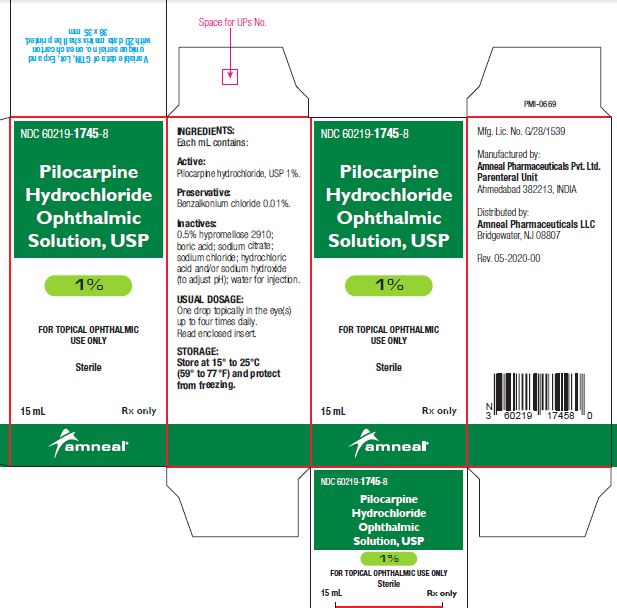
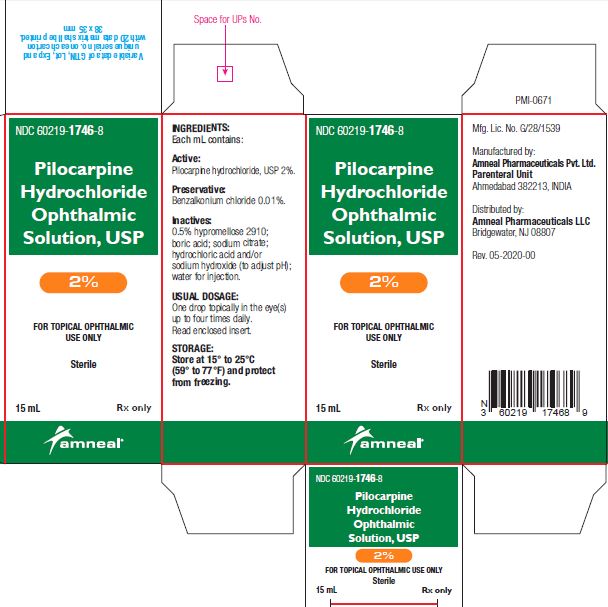
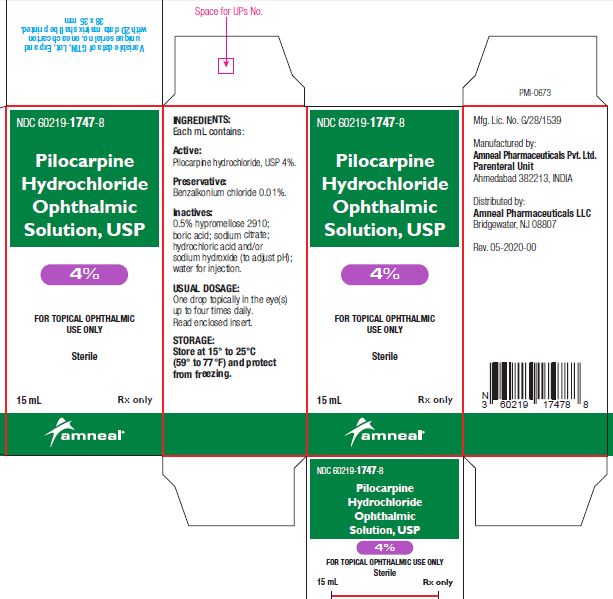
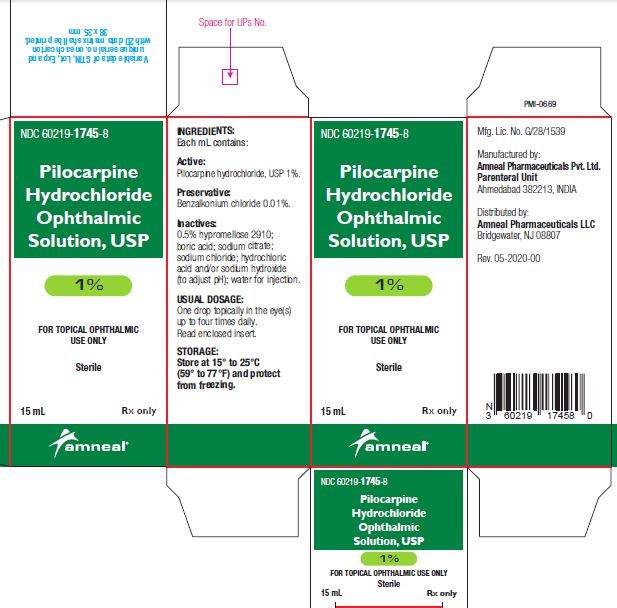
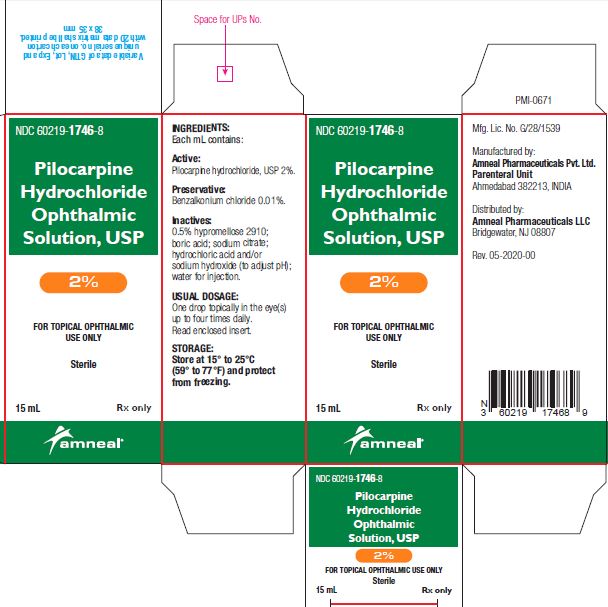
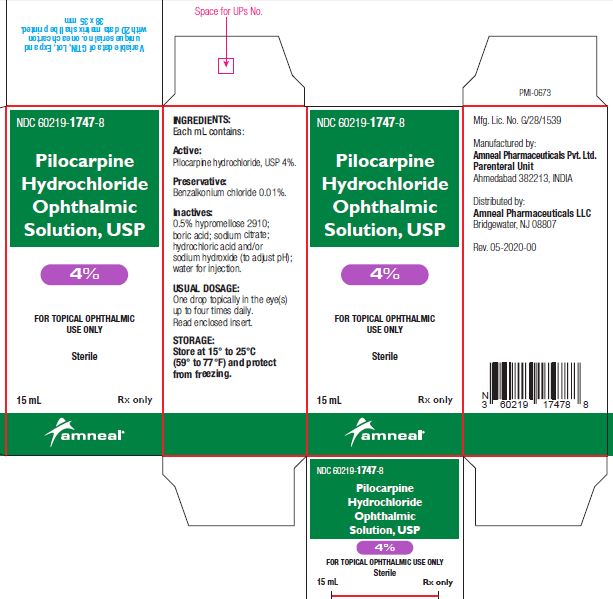
3 DOSAGE FORMS AND STRENGTHSBottle filled with 15 mL of 1% (10 mg/mL), 2% (20 mg/mL) or 4% (40 mg/mL) pilocarpine hydrochloride, USP sterile ophthalmic solution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Poor Illumination - Patients should be advised to exercise caution in night driving and other hazardous occupations in poor illumination. In addition, miotics may cause accommodative spasm ...
-
6 ADVERSE REACTIONSClinical Studies Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C. Animal reproduction studies have not been conducted with pilocarpine hydrochloride. It is also not known whether pilocarpine hydrochloride can cause fetal ...
-
10 OVERDOSAGESystemic toxicity following topical ocular administration of pilocarpine is rare, but occasionally patients who are sensitive may develop sweating and gastrointestinal overactivity following the ...
-
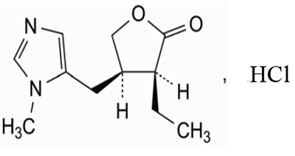
11 DESCRIPTIONPilocarpine hydrochloride ophthalmic solution, USP is a cholinergic agonist prepared as a sterile topical ophthalmic solution. The active ingredient is represented by the chemical ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pilocarpine hydrochloride is a direct acting cholinergic parasympathomimetic agent which acts through direct stimulation of muscarinic receptors and smooth muscle such ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There have been no long-term studies done using pilocarpine hydrochloride in animals to evaluate carcinogenic potential.
-
14 CLINICAL STUDIESIn clinical trials reported in the medical literature, pilocarpine ophthalmic solution reduced intraocular pressure (IOP) by 3 mm Hg to 7 mm Hg in patients with open-angle glaucoma. Pilocarpine ...
-
16 HOW
SUPPLIED/STORAGE AND HANDLINGPilocarpine Hydrochloride Ophthalmic Solution USP, 1%, 2% and 4% is supplied sterile in natural low density polyethylene plastic bottles and natural low density polyethylene tips with dark green ...
-
17 PATIENT COUNSELING INFORMATION17.1 - Avoiding Contamination of the Product - Do not touch dropper tip to any surface, as this may contaminate the contents. 17.2 - Night Driving - Caution is advised with night driving and when ...
-
PRINCIPAL DISPLAY PANELNDC 60219-1745-8 - Pilocarpine hydrochloride ophthalmic solution USP, 1% Rx only - Amneal Pharmaceuticals LLC - NDC 60219-1746-8 - Pilocarpine hydrochloride ophthalmic ...
-
INGREDIENTS AND APPEARANCEProduct Information