Label: HYDROCHLOROTHIAZIDE capsule, gelatin coated
- NDC Code(s): 59746-382-05, 59746-382-06, 59746-382-10
- Packager: JUBILANT CADISTA PHARMACEUTICALS INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 5, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
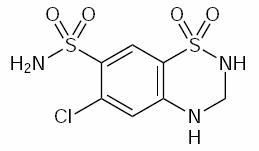
DESCRIPTIONHydrochlorothiazide capsules, USP 12.5 mg is the 3,4-dihydro derivative of chlorothiazide. Its chemical name is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its ...
-
CLINICAL PHARMACOLOGYHydrochlorothiazide blocks the reabsorption of sodium and chloride ions, and it thereby increases the quantity of sodium traversing the distal tubule and the volume of water excreted. A portion of ...
-
INDICATIONS AND USAGEHydrochlorothiazide capsules are indicated in the management of hypertension either as the sole therapeutic agent, or in combination with other antihypertensives. Unlike potassium sparing ...
-
CONTRAINDICATIONSHydrochlorothiazide is contraindicated in patients with anuria. Hypersensitivity to this product or other sulfonamide derived drugs is also contraindicated.
-
WARNINGSAcute Myopia and Secondary Angle-Closure Glaucoma: Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma ...
-
PRECAUTIONSElectrolyte and Fluid Balance Status: In published studies, clinically significant hypokalemia has been consistently less common in patients who received 12.5 mg of hydrochlorothiazide than in ...
-
Drug Interactions:When given concurrently the following drugs may interact with thiazide diuretics: Alcohol, barbiturates, or narcotics: potentiation of orthostatic hypotension may occur. Antidiabetic drugs ...
-
Pregnancy:Teratogenic Effects: Studies in which hydrochlorothiazide was orally administered to pregnant mice and rats during their respective periods of major organogenesis at doses up to 3,000 and 1,000 ...
-
Pediatric Use:Safety and effectiveness in pediatric patients have not been established.
-
Elderly Use:A greater blood pressure reduction and an increase in side effects may be observed in the elderly (i.e., > 65 years) with hydrochlorothiazide. Starting treatment with the lowest available dose of ...
-
ADVERSE REACTIONSThe adverse reactions associated with hydrochlorothiazide have been shown to be dose related. In controlled clinical trials, the adverse events reported with doses of 12.5 mg hydrochlorothiazide ...
-
OVERDOSAGEThe most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has ...
-
DOSAGE AND ADMINISTRATIONFor Control of Hypertension: The adult initial dose of hydrochlorothiazide is one capsule given once daily whether given alone or in combination with other antihypertensives. Total daily doses ...
-
HOW SUPPLIEDHydrochlorothiazide capsules USP, 12.5 mg are Size 4 capsules, light blue opaque cap and white opaque body with - "TL 382" axially printed in black ink on cap and body. Bottles of 30 with ...
-
PACKAGE LABELNDC 59746-382-06 - Hydrochlorothiazide Capsules, USP - 12.5 mg - CADISTA™ 100 Capsules - Rx Only - Each capsule contains: Hydrochlorothiazide USP...12.5 mg - Dispense in a tight ...
-
INGREDIENTS AND APPEARANCEProduct Information