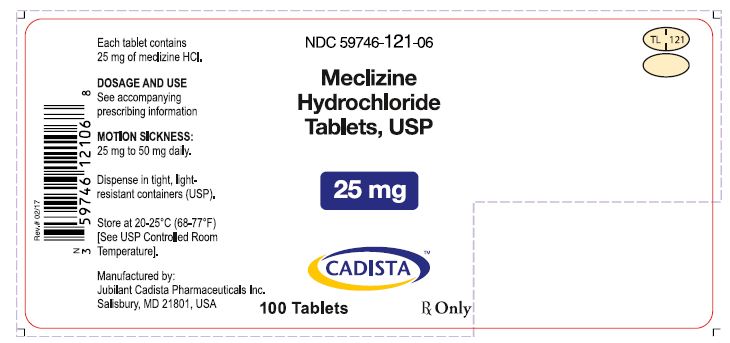
Label: MECLIZINE HYDROCHLORIDE tablet
MECLIZINE HYDROCHLORIDE- meclizine hydrocloride tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 59746-121-06, 59746-121-10, 59746-122-06, 59746-122-10 - Packager: JUBILANT CADISTA PHARMACEUTICALS INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 1, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MECLIZINE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for MECLIZINE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMeclizine hydrochloride is indicated for the treatment of vertigo associated with diseases affecting the vestibular system in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage is 25 mg to 100 mg daily administered orally, in divided doses, depending upon clinical response. 2.2 Administration ...
-
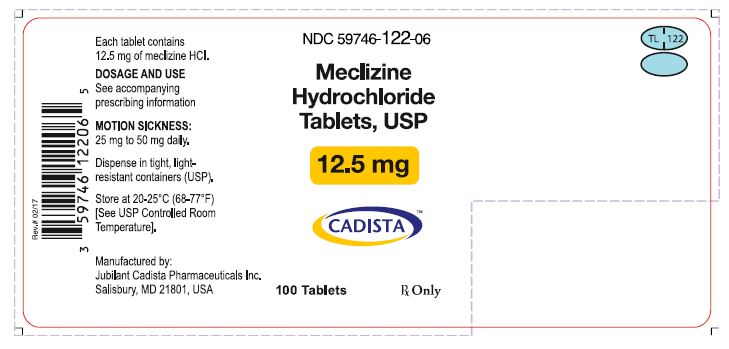
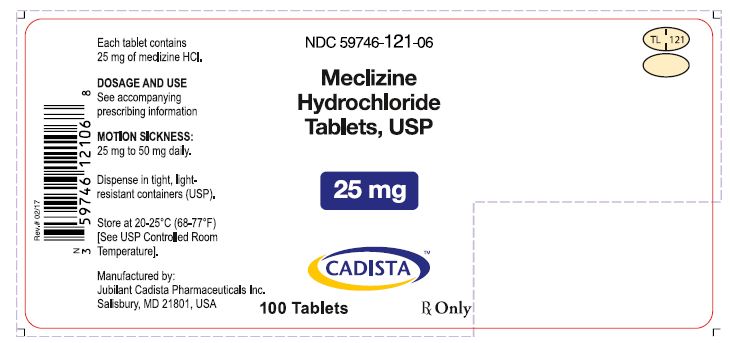
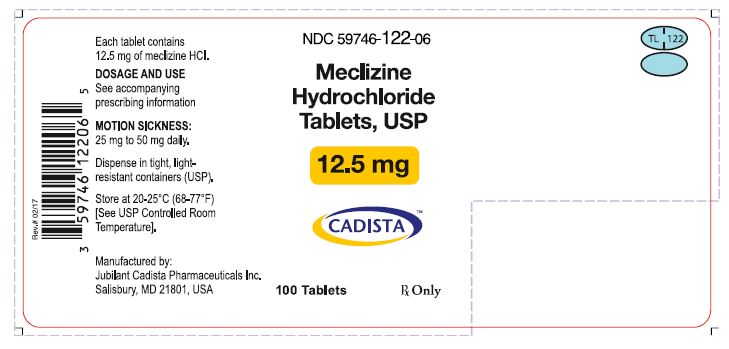
3 DOSAGE FORMS AND STRENGTHSMeclizine hydrochloride tablets USP, 12.5 mg (Blue, oval shaped tablets, debossed with “TL 122” with score on one side and plain on the other side.) Meclizine hydrochloride tablets USP, 25 mg ...
-
4 CONTRAINDICATIONSMeclizine hydrochloride is contraindicated in patients with a hypersensitivity to meclizine or any of the inactive ingredients [see Adverse Reactions (6) and Description (11)].
-
5 WARNINGS AND PRECAUTIONS5.1 Drowsiness - Since drowsiness may occur with use of meclizine hydrochloride, patients should be warned of this possibility and cautioned against driving a car or operating dangerous ...
-
6 ADVERSE REACTIONSThe following adverse reactions associated with the use of meclizine hydrochloride were identified in clinical studies or postmarketing reports. Because some of these reactions were reported ...
-
7 DRUG INTERACTIONS7.1 CNS Depressants - There may be increased CNS depression when meclizine hydrochloride is administered concurrently with other CNS depressants, including alcohol [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Data from epidemiological studies have not generally indicated a drug-associated risk of major birth defects with meclizine during pregnancy. However, in a ...
-
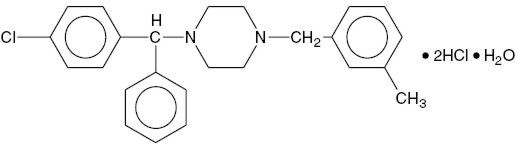
11 DESCRIPTIONMeclizine hydrochloride, USP a histamine (H1) receptor antagonist, is a white or slightly yellowish, crystalline powder. It has the following structural formula: Chemically, meclizine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism by which meclizine exerts its therapeutic effect is unknown but is presumed to involve antagonism of the histamine H1 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Animal studies to assess the carcinogenic potential of meclizine have not been conducted. Mutagenesis - Genetic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Meclizine hydrochloride tablets, USP are available in the following strengths and package sizes: 12.5 mg (Blue, oval shaped tablets, debossed with “TL 122” with score on one ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions - Advise patients that the tablets must be swallowed whole [see Dosage and Administration (2.1)]. Adverse Reactions - Advise patients that meclizine hydrochloride may ...
-
PRINCIPAL DISPLAY PANELNDC 59746-122-06 - Meclizine Hydrochloride Tablets, USP - 12.5 mg - CADISTA™ 100 Tablets - Rx Only - Each tablet Contains 12.5 mg of meclizine HCl - DOSAGE AND USE - See accompanying ...
-
INGREDIENTS AND APPEARANCEProduct Information