Label: DICLOXACILLIN SODIUM capsule
- NDC Code(s): 59651-565-01, 59651-566-01
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug resistant bacteria and maintain the effectiveness of dicloxacillin sodium capsules and other antibacterial drugs, dicloxacillin sodium capsules should be used only ...
-
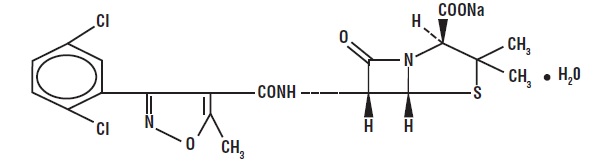
DESCRIPTIONDicloxacillin sodium, USP is an antibacterial agent of the isoxazolyl penicillin series. It is a penicillinase resistant, acid resistant semisynthetic penicillin suitable for oral ...
-
CLINICAL PHARMACOLOGYMicrobiology - Mechanism of Action - Penicillinase-resistant penicillins exert a bactericidal action against penicillin-susceptible microorganisms during the state of active multiplication ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of dicloxacillin sodium capsules and other antibacterial drugs, dicloxacillin sodium capsules should be used ...
-
CONTRAINDICATIONSDicloxacillin sodium capsules are contraindicated in persons who have shown hypersensitivity to any of the penicillins or any component of the formulations.
-
WARNINGSSerious and occasionally fatal hypersensitivity (anaphylactic shock with collapse) reactions have occurred in patients receiving penicillin. The incidence of anaphylactic shock in all ...
-
PRECAUTIONSGeneral - Prescribing dicloxacillin sodium capsules in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient ...
-
ADVERSE REACTIONSHypersensitive Reactions - The reported incidence of allergic reactions to penicillin ranges from 0.7% to 10% (see WARNINGS). Sensitization is usually the result of treatment, but some ...
-
DOSAGE AND ADMINISTRATIONConcurrent administration of the penicillinase-resistant penicillins and probenecid increases and prolongs serum penicillin levels. Probenecid decreases the apparent volume of distribution and ...
-
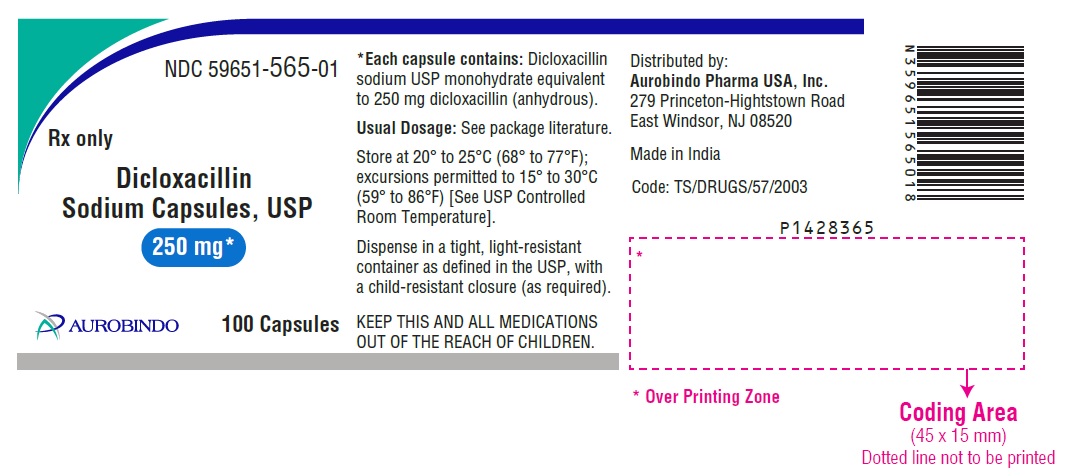
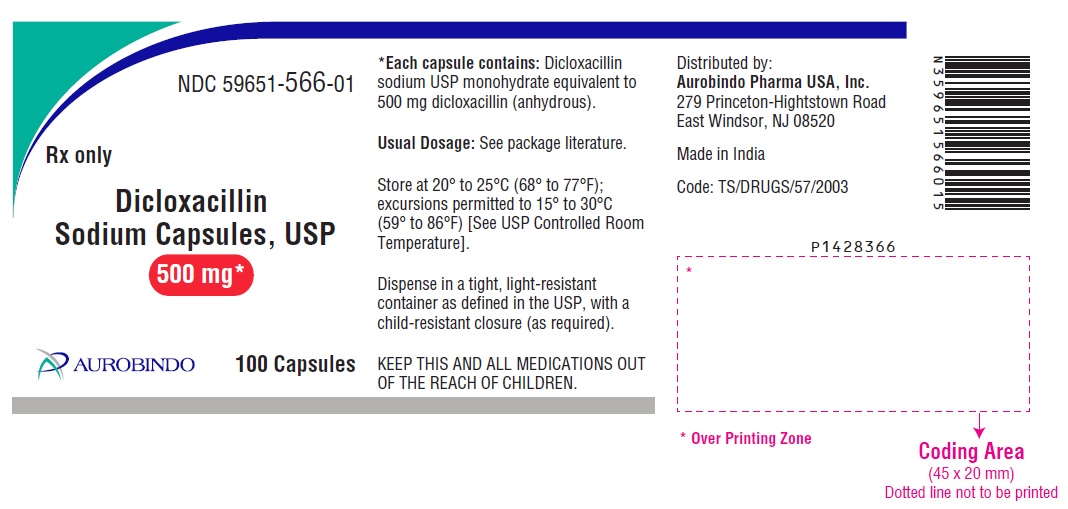
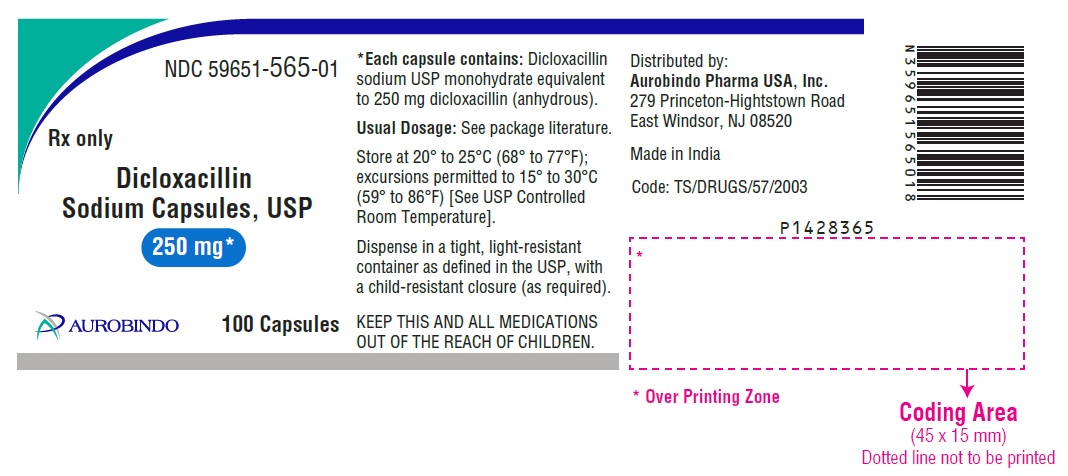
HOW SUPPLIEDDicloxacillin Sodium Capsules USP are available as follows: 250 mg: Each capsule contains dicloxacillin sodium USP monohydrate equivalent to 250 mg dicloxacillin (anhydrous), with blue opaque ...
-
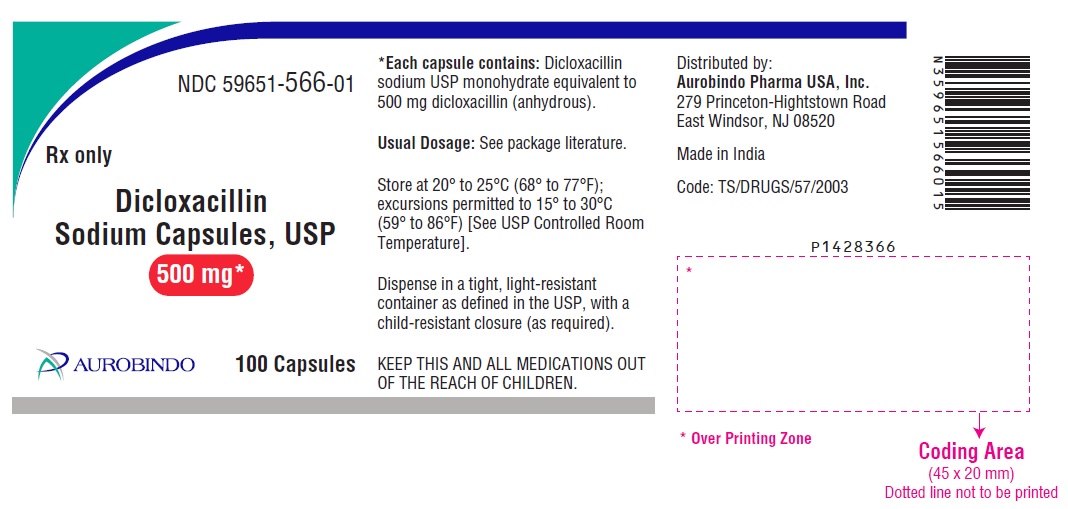
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 250 mg (100 Capsules Bottle)NDC 59651-565-01 - Rx only - Dicloxacillin - Sodium Capsules, USP - 250 mg* AUROBINDO 100 Capsules - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 500 mg (100 Capsules Bottle) NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information