Label: PREDNISONE tablet
- NDC Code(s): 59651-484-01, 59651-484-78, 59651-484-99
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
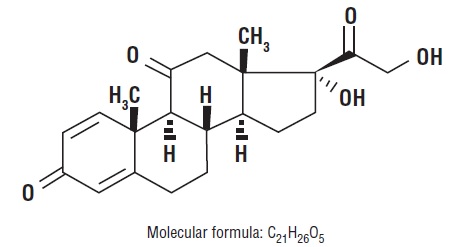
DESCRIPTIONGlucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, that are readily absorbed from the gastrointestinal tract. The formula for prednisone is C21H26O5. Chemically ...
-
ACTIONSNaturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic ...
-
INDICATIONSEndocrine disorders: primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice; synthetic analogs may be used in conjunction with mineralocorticoids where ...
-
CONTRAINDICATIONSCONTRAINDICATIONS: Prednisone tablets are contraindicated in systemic fungal infections.
-
WARNINGSWARNINGS: In patients on corticosteroid therapy subject to any unusual stress, increased dosage of rapidly acting corticosteroids before, during and after the stressful situation is indicated ...
-
PRECAUTIONSPRECAUTIONS: Information for Patients: Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles. Patients should also be advised ...
-
ADVERSE REACTIONSFluid and electrolyte disturbances: sodium retention, fluid retention, congestive heart failure in susceptible patients, potassium loss, hypokalemic alkalosis, hypertension. Musculoskeletal ...
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION: Dosage of prednisone tablets should be individualized according to the severity of the disease and the response of the patient. For infants and children, the recommended ...
-
HOW SUPPLIEDPrednisone Tablets USP, 1 mg are beige colored, round, biconvex tablets, scored on one side and “PI”, “1” on other side. They are supplied as follows: Bottles of 100 NDC ...
-
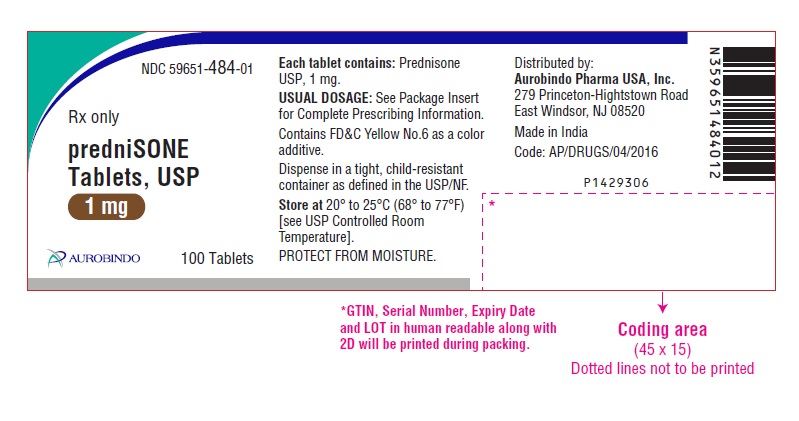
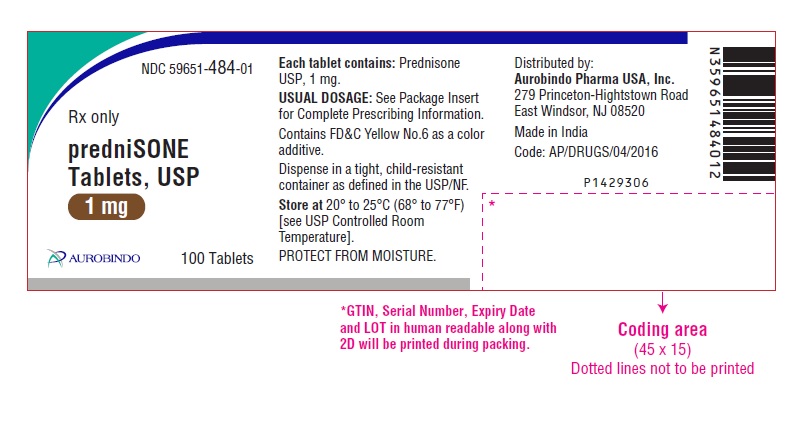
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg (100 Tablet Bottle)NDC 59651-484-01 - Rx only - predniSONE - Tablets, USP - 1 mg - AUROBINDO 100 Tablets
-
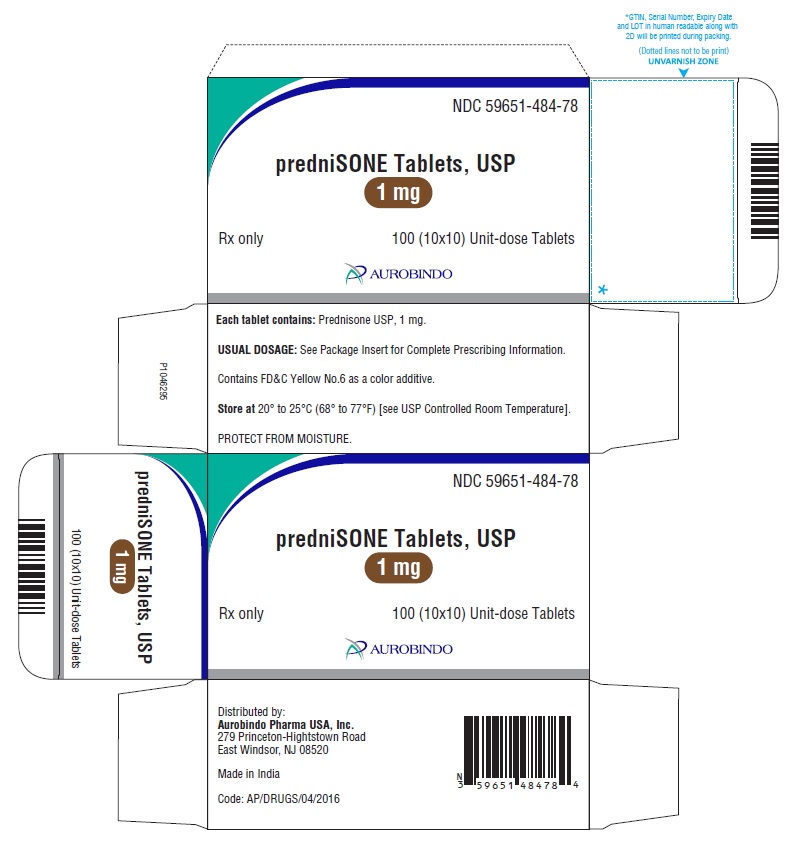
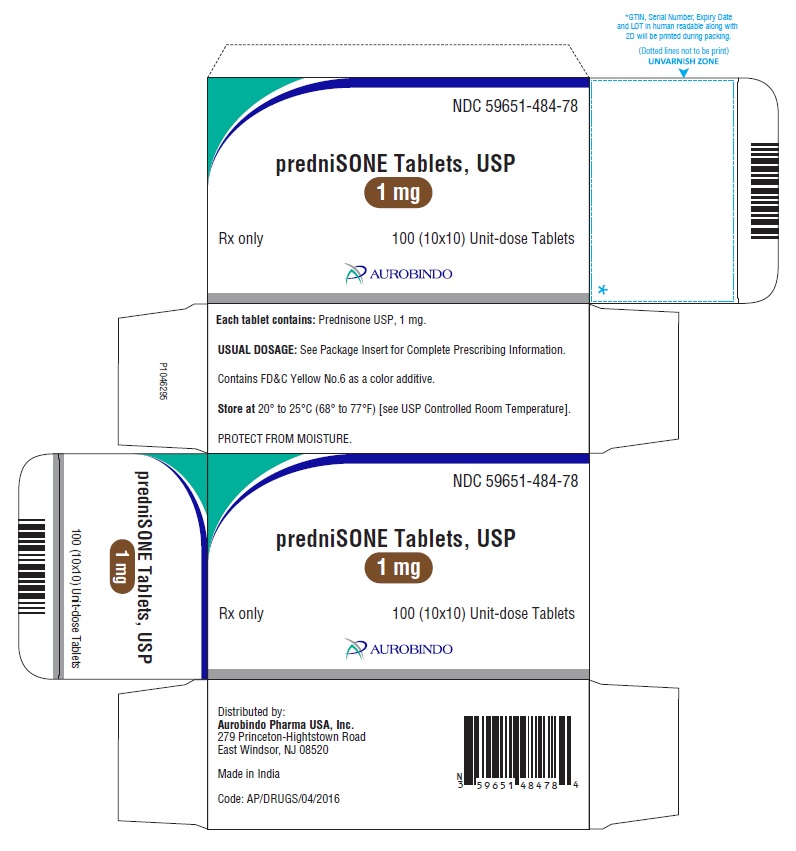
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg 100(10x10) Unit-dose TabletsNDC 59651-484-78 - predniSONE Tablets, USP - 1 mg - Rx only 100 (10x10) Unit-dose Tablets - AUROBINDO
-
INGREDIENTS AND APPEARANCEProduct Information