Label: SPIRONOLACTONE tablet
- NDC Code(s): 59651-426-01, 59651-426-05, 59651-426-99, 59651-427-01, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SPIRONOLACTONE TABLETS safely and effectively. See full prescribing information for SPIRONOLACTONE TABLETS. SPIRONOLACTONE tablets ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Heart Failure - Spironolactone tablets are indicated for treatment of NYHA Class III-IV heart failure and reduced ejection fraction to increase survival, manage edema, and reduce the need for ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - Spironolactone tablets can be taken with or without food, but should be taken consistently with respect to food [see Clinical Pharmacology (12.3)]. 2.2 Treatment ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 25 mg white to almost white, round shaped, flat beveled edge, uncoated tablet debossed with "SPIT" on one side and "25" on the other side. Tablets: 50 mg white to almost white, round ...
-
4 CONTRAINDICATIONSSpironolactone tablets are contraindicated in the patients with: Hyperkalemia - Addison’s disease - Concomitant use of eplerenone
-
5 WARNINGS AND PRECAUTIONS5.1 Hyperkalemia - Spironolactone can cause hyperkalemia. This risk is increased by impaired renal function or concomitant potassium supplementation, potassium-containing salt substitutes or ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hyperkalemia [see Warnings and Precautions (5.1)] Hypotension and Worsening Renal Function [see ...
-
7 DRUG INTERACTIONS7.1 Drugs and Supplements Increasing Serum Potassium - Concomitant administration of spironolactone with potassium supplementation or drugs that can increase potassium may lead to severe ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on mechanism of action and findings in animal studies, spironolactone may affect sex differentiation of the male during embryogenesis (see Data). Rat ...
-
10 OVERDOSAGEThe oral LD50 of spironolactone is greater than 1000 mg/kg in mice, rats, and rabbits. Acute overdosage of spironolactone may be manifested by drowsiness, mental confusion, maculopapular or ...
-
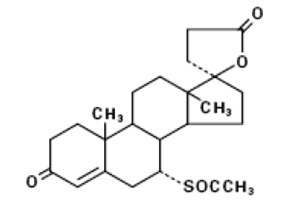
11 DESCRIPTIONSpironolactone oral tablets contain 25 mg, 50 mg, or 100 mg of the aldosterone antagonist spironolactone, 17-hydroxy-7α-mercapto-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone acetate, which ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Spironolactone and its active metabolites are specific pharmacologic antagonists of aldosterone, acting primarily through competitive binding of receptors at the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Orally administered spironolactone has been shown to be a tumorigen in dietary administration studies performed in ...
-
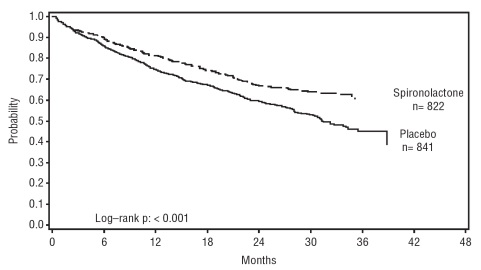
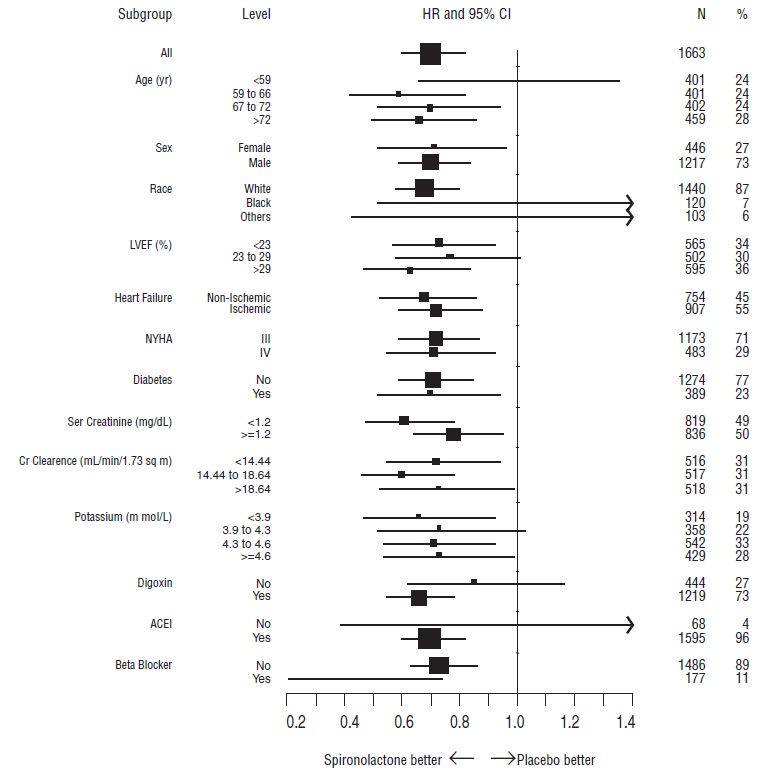
14 CLINICAL STUDIES14.1 Heart Failure - The Randomized Spironolactone Evaluation Study was a placebo controlled, double-blind study of the effect of spironolactone on mortality in patients with highly symptomatic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSpironolactone Tablets USP, 25 mg are white to almost white, round shaped, flat beveled edge, uncoated tablet debossed with "SPIT" on one side and "25" on the other side, supplied as: Bottle of ...
-
17 PATIENT COUNSELING INFORMATIONPatients who receive spironolactone should be advised to avoid potassium supplements and foods containing high levels of potassium, including salt substitutes. Distributed by: Aurobindo Pharma ...
-
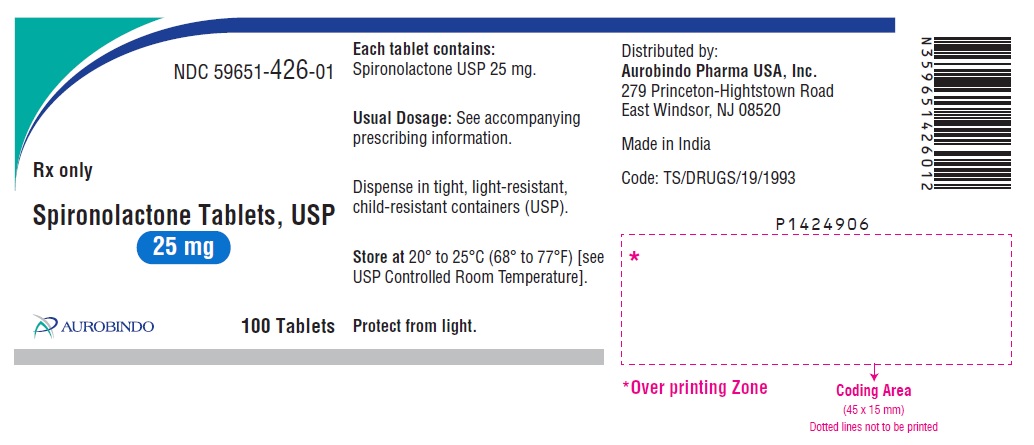
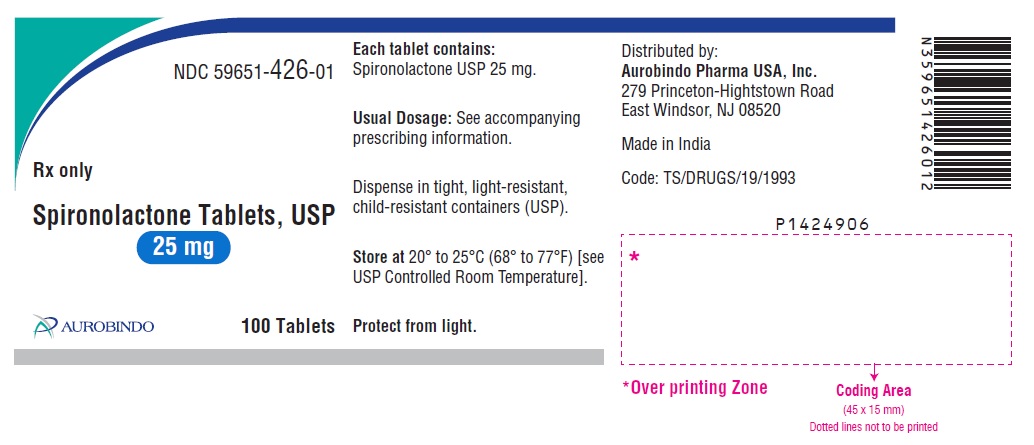
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 25 mg (100 Tablets Bottle)NDC 59651-426-01 - Rx only - Spironolactone Tablets, USP - 25 mg - AUROBINDO 100 Tablets
-
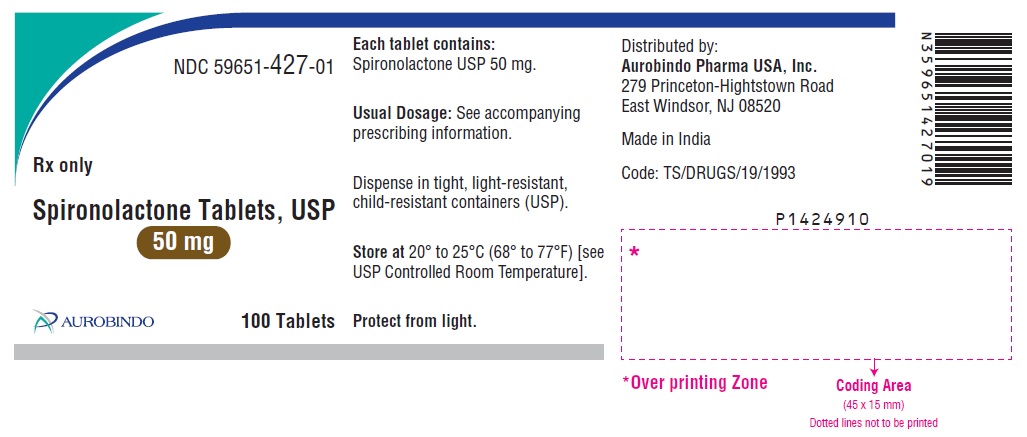
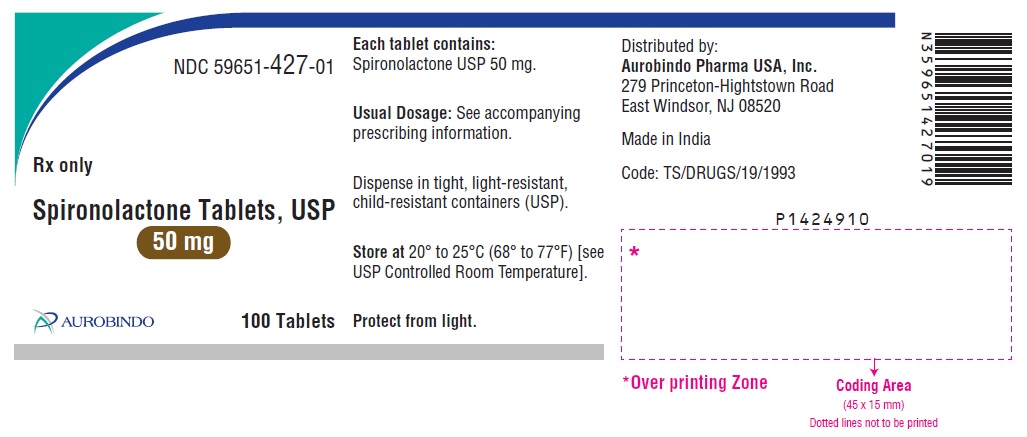
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 mg (100 Tablets Bottle)NDC 59651-427-01 - Rx only - Spironolactone Tablets, USP - 50 mg - AUROBINDO 100 Tablets
-
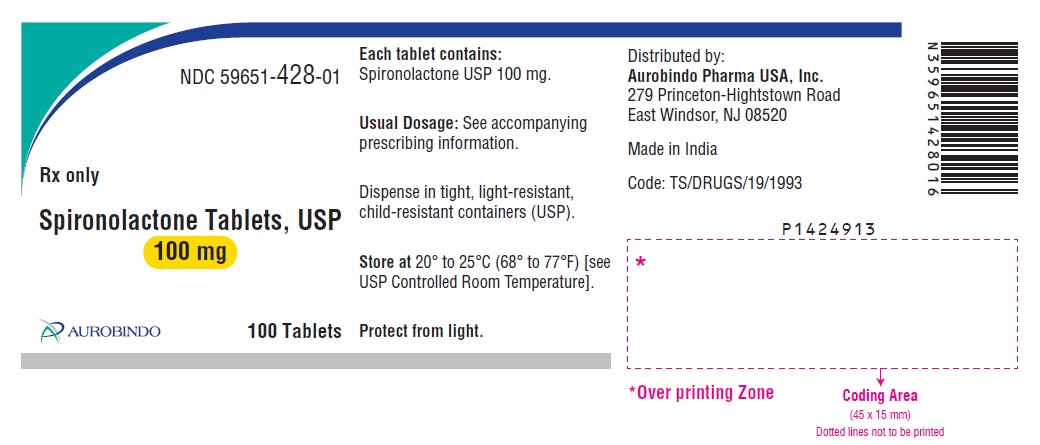
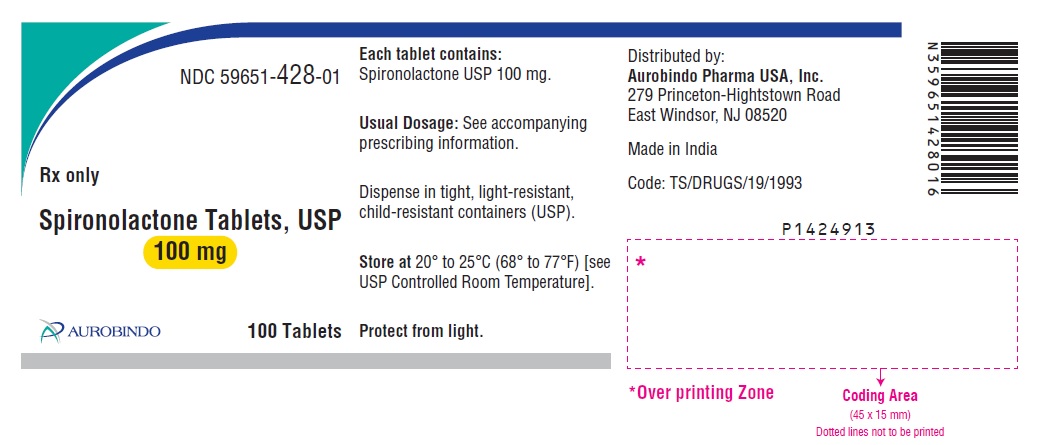
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 100 mg (100 Tablets Bottle)NDC 59651-428-01 - Rx only - Spironolactone Tablets, USP - 100 mg - AUROBINDO 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information