ZAFIRLUKAST TABLETS
-
(za FIR loo kast)
Read the Patient Information leaflet before you start taking zafirlukast tablets and each time you get a refill. There may be new information. This ...
ZAFIRLUKAST TABLETS
(za FIR loo kast)
Read the Patient Information leaflet before you start taking zafirlukast tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What are zafirlukast tablets?
Zafirlukast tablets are a prescription medicine used to help prevent asthma attacks and for the long-term treatment of asthma symptoms in adults and children 5 years and older.
It is not known if zafirlukast tablets are safe and effective when used in children under 5 years old. The effect of zafirlukast tablets on growth in children has not been determined.
Do not take zafirlukast tablets if you need relief right away for a sudden asthma attack. If you get an asthma attack, you should follow the instructions your healthcare provider gave you for treating asthma attacks.
Who should not take zafirlukast tablets?
Do not take zafirlukast tablets if you;
- are allergic to zafirlukast or any of the ingredients in zafirlukast tablets. See the end of this leaflet for a complete list of ingredients in zafirlukast tablets.
- have problems with your liver.
What should I tell my healthcare provider before taking zafirlukast tablets?
Before you take zafirlukast tablets, tell your healthcare provider if you:
- have liver problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if zafirlukast tablets will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. Zafirlukast can pass into your milk; it is not known whether zafirlukast tablets may harm your baby. Women who are breastfeeding should not take zafirlukast tablets.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Zafirlukast tablets may affect the way other medicines work, and other medicines may affect how zafirlukast tablets works.
Especially tell your healthcare provider if you take:
- warfarin sodium (Coumadin, Jantoven)
- erythromycin (ERYC, ERY-TAB, PCE)
- theophylline (Elixophyllin, Theo-24, Theochron, Theolair, Uniphyl)
- fluconazole (Diflucan)
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take zafirlukast tablets?
- Take zafirlukast tablets exactly as your healthcare provider tells you to take it.
- Take zafirlukast tablets regularly, even if you do not have asthma symptoms. Do not change your dose or stop taking zafirlukast tablets without talking to your healthcare provider.
- Do not stop taking or change the dose of your other asthma medicines unless your healthcare provider tells you to.
- Take your prescribed dose of zafirlukast tablets by mouth at least 1 hour before or 2 hours after meals.
- Zafirlukast tablets does not treat the symptoms of a sudden asthma attack. Always have a short-acting beta2-agonist medicine (rescue inhaler) with you to treat sudden symptoms. If you do not have a rescue inhaler medicine, talk to your healthcare provider to have one prescribed for you.
- If you take too much zafirlukast, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of zafirlukast tablets?
Zafirlukast tablets may cause serious side effects, including:
-
Severe liver problems. In some cases, these liver problems can lead to liver failure, the need for a liver transplant or death. Tell your healthcare provider right away if you have:
- pain or tenderness in the right upper side of your stomach area (abdomen)
- nausea
- tiredness
- itchiness
- yellowing of your skin or the whites of your eyes
- flu-like symptoms
- loss of appetite
- dark (tea colored) urine
-
Inflammation of your blood vessels. Rarely, this can happen in people with asthma who take zafirlukast tablets. This usually, but not always, happens in people who also take a steroid medicine by mouth that is being stopped or the dose is being lowered. Tell your healthcare provider right away if you have:
- a feeling of pins and needles or numbness of your arms or legs
- flu like symptoms
- rash
- pain and swelling of your sinuses
-
Changes in behaviour or mood. Tell your healthcare provider if you have changes in your behaviour, problems sleeping or feel very sad.
-
Hypersensitivity reactions. Tell your healthcare provider if you have severe itching, breathing problems, skin rash, skin blisters, or skin redness, or swelling.
The most common side effects of zafirlukast tablets in people 12 years and older include:
- headache
- infection
- nausea
- diarrhea
- pain (generalized)
The most common side effects of zafirlukast tablets in children 5 to 11 years include:
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of zafirlukast tablets. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Aurobindo Pharma USA, Inc. at 1-866-850-2876.
How should I store zafirlukast tablets?
- Store zafirlukast tablets at 20° to 25°C (68° to 77°F)
- Keep zafirlukast tablets dry.
- Keep zafirlukast tablets in a tight closed container and keep zafirlukast tablets out of the light.
- Keep zafirlukast tablets and all medicines out of the reach of children.
General information about the safe and effective use of zafirlukast tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use zafirlukast tablets for a condition for which it was not prescribed. Do not give zafirlukast tablets to other people, even if they have the same symptoms that you have. They may harm them.
This Patient Information leaflet summarizes the most important information about zafirlukast tablets. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about zafirlukast tablets that is written for healthcare professionals.
For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876.
What are the ingredients in zafirlukast tablets?
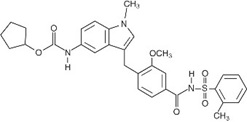
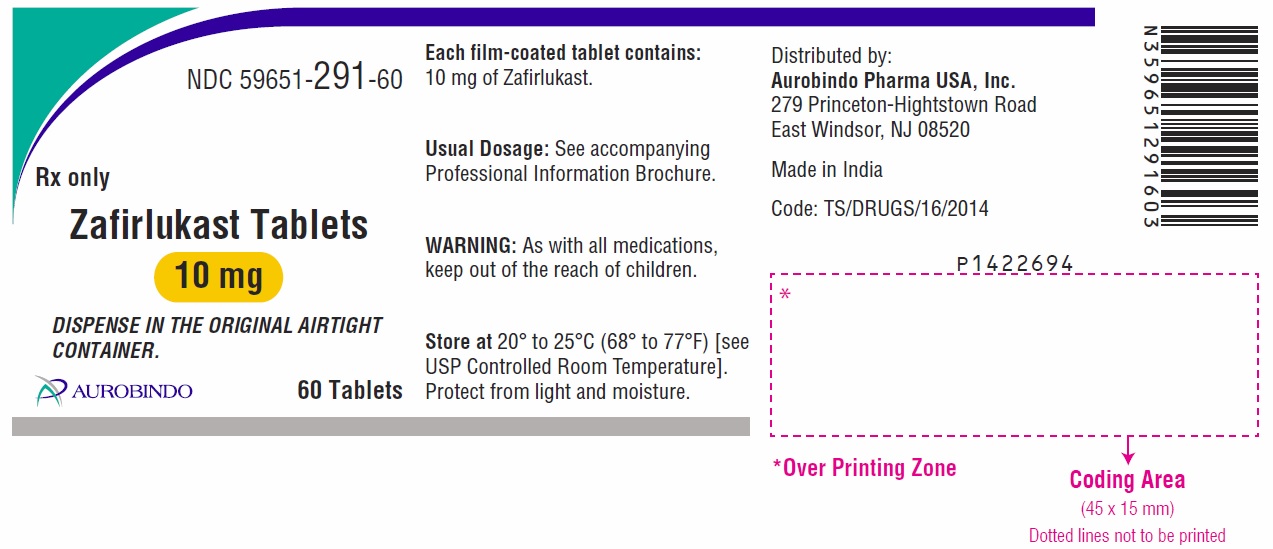
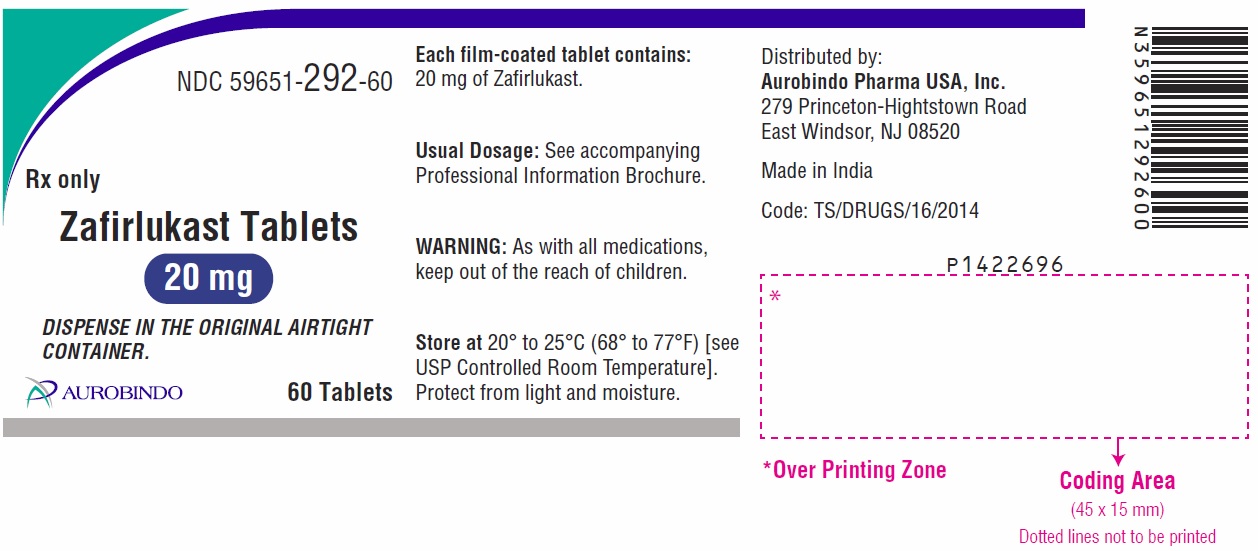
Active ingredient: zafirlukast
Inactive ingredients: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone and titanium dioxide.
What do zafirlukast tablets look like?
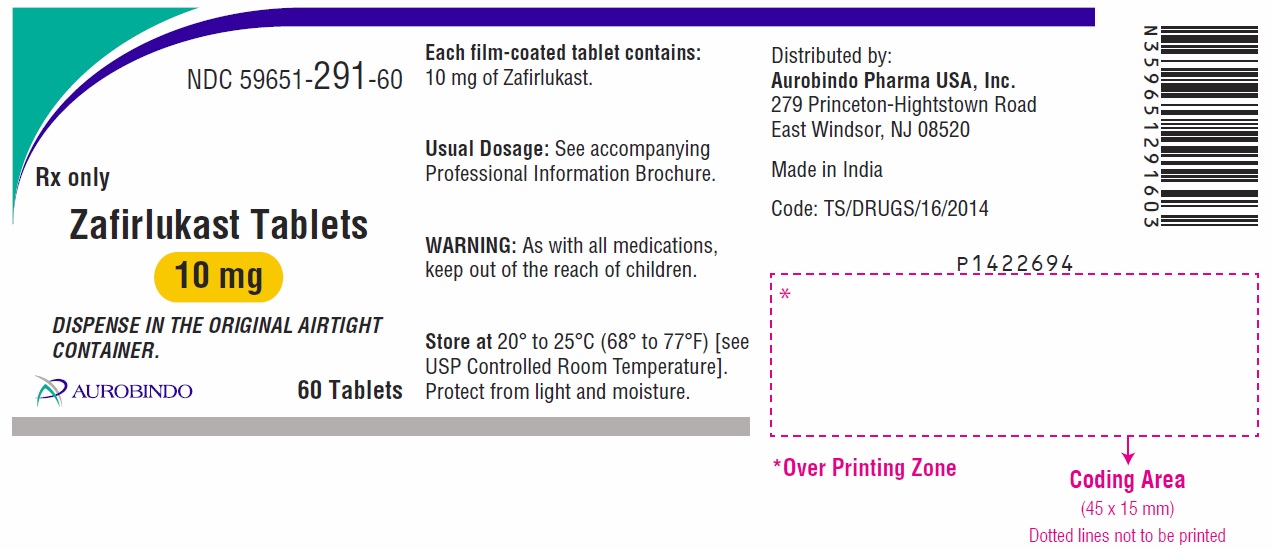
- the 10 mg tablet is white, round, biconvex, film-coated tablets debossed with “ 1” on one side and “B” on other side
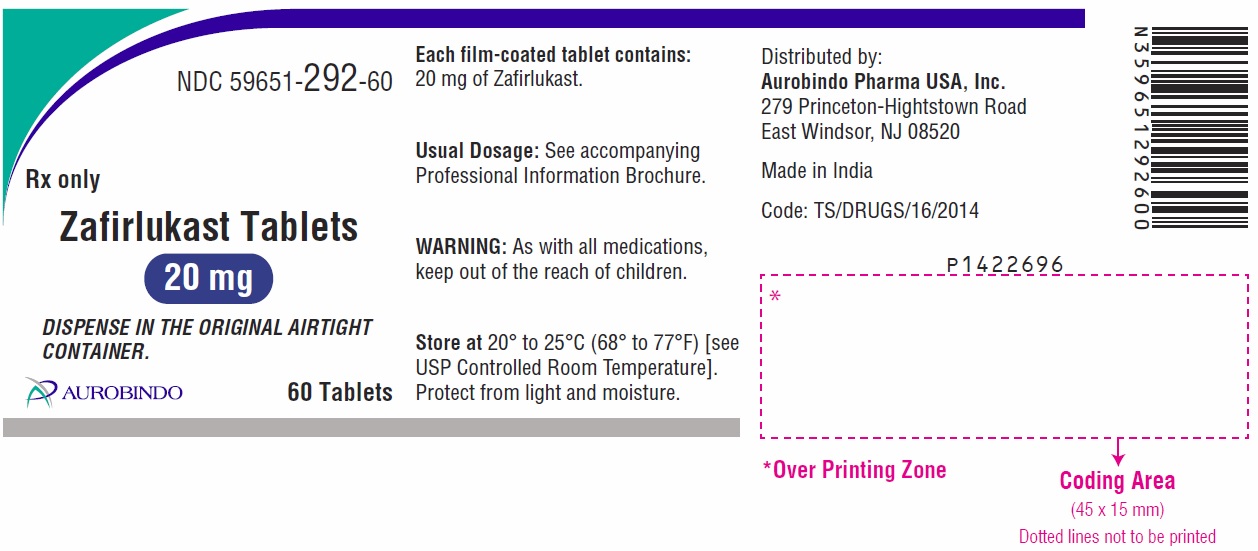
- the 20 mg tablet is white, round, biconvex, film-coated tablets debossed with “ 2” on one side and “B” on other side.
The brands listed are trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Issued: June 2023
Close