Label: PILOCARPINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 59651-224-01, 59651-225-01
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
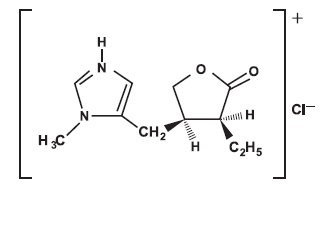
DESCRIPTIONDESCRIPTION - Pilocarpine hydrochloride tablets, USP contain pilocarpine hydrochloride, a cholinergic agonist for oral use. Pilocarpine hydrochloride is a white or almost white, crystalline ...
-
CLINICAL PHARMACOLOGYPharmacodynamics: Pilocarpine is a cholinergic parasympathomimetic agent exerting a broad spectrum of pharmacologic effects with predominant muscarinic action. Pilocarpine, in appropriate ...
-
INDICATIONS & USAGEINDICATIONS AND USAGE - Pilocarpine hydrochloride tablets are indicated for 1) the treatment of symptoms of dry mouth from salivary gland hypofunction caused by radiotherapy for cancer of the ...
-
CONTRAINDICATIONS - Pilocarpine hydrochloride tablets are contraindicated in patients with uncontrolled asthma, known hypersensitivity to pilocarpine, and when miosis is undesirable, e.g., in ...
-
WARNINGS - Cardiovascular Disease: Patients with significant cardiovascular disease may be unable to compensate for transient changes in hemodynamics or rhythm induced by pilocarpine ...
-
PRECAUTIONSGeneral - Pilocarpine toxicity is characterized by an exaggeration of its parasympathomimetic effects. These may include: headache, visual disturbance, lacrimation, sweating, respiratory ...
-
ADVERSE REACTIONSHead & Neck Cancer Patients: In controlled studies, 217 patients received pilocarpine, of whom 68% were men and 32% were women. Race distribution was 91% Caucasian, 8% Black, and 1% of other ...
-
OVERDOSAGEMANAGEMENT OF OVERDOSE - Fatal overdosage with pilocarpine has been reported in the scientific literature at doses presumed to be greater than 100 mg in two hospitalized patients. 100 mg of ...
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION - Regardless of the indication, the starting dose in patients with moderate hepatic impairment should be 5 mg twice daily, followed by adjustment based on therapeutic ...
-
HOW SUPPLIEDPilocarpine Hydrochloride Tablets, USP 5 mg are off white to white colour, biconvex round shaped film-coated tablet debossed with “PIL” on one side and “5” on the other side. Each tablet contains ...
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 5 mg (100 Tablets Bottle)NDC 59651-224-01 - Rx only - Pilocarpine Hydrochloride - Tablets, USP - 5 mg - Aurobindo 100 Tablets
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 7.5 mg (100 Tablets Bottle)NDC 59651-225-01 - Rx only - Pilocarpine Hydrochloride - Tablets, USP - 7.5 mg - Aurobindo 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information