See FDA Approved Patient Labeling (Patient Information)
Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other Vasospastic Reactions, and Cerebrovascular Events
-
Inform patients that ...
See FDA Approved Patient Labeling (Patient Information)
Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other Vasospastic Reactions, and Cerebrovascular Events
Inform patients that eletriptan hydrobromide may cause serious cardiovascular adverse reactions such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious cardiovascular reactions can occur without warning symptoms, instruct patients to be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and instruct them to ask for medical advice when observing any indicative sign or symptoms. Instruct patients to seek medical advice if they have symptoms of other vasospastic reactions [see Warnings and Precautions (5.1, 5.2, 5.4, 5.5, and 5.8)].
Anaphylactic/Anaphylactoid Reactions
Inform patients that anaphylactic/anaphylactoid reactions have occurred in patients receiving eletriptan hydrobromide. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens [see Contraindications (4)].
Medication Overuse Headache
Inform patients that use of drugs to treat acute migraines for 10 or more days per month may lead to an exacerbation of headache, and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary) [see Warnings and Precautions (5.6)].
Serotonin Syndrome
Inform patients about the risk of serotonin syndrome with the use of eletriptan hydrobromide or other triptans, particularly during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors (SNRIs) [see Warnings and Precautions (5.7) and Drug Interactions (7.3)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during therapy [see Use in Specific Populations (8.1)].
Lactation
Inform patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed [see Use in Specific Populations (8.2)].
Patient Information
Eletriptan Hydrobromide Tablets
(EL-e-TRIP-tan HYE-droe-BROE-mide)
Please read this information before you start taking eletriptan hydrobromide tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important information I should know about eletriptan hydrobromide tablets?
Eletriptan hydrobromide tablets can cause serious side effects, including:
Heart attack and other heart problems. Heart problems may lead to death.
Stop taking eletriptan hydrobromide tablets and get emergency medical help right away if you have any of the following symptoms of a heart attack:
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- chest pain or chest discomfort that feels like an uncomfortable heavy pressure, squeezing, fullness, or pain
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
Eletriptan hydrobromide tablets are not for people with risk factors for heart disease unless a heart exam is done and shows no problem. You have a higher risk for heart disease if you:
- have high blood pressure
- have high cholesterol levels
- smoke
- are overweight
- have diabetes
- have a family history of heart disease
- are a female who has gone through menopause
- are a male over age 40
Serotonin syndrome. Serotonin syndrome is a serious and life-threatening problem that can happen in people taking eletriptan hydrobromide tablets, especially if eletriptan hydrobromide tablets are taken with anti-depressant medicines called selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors (SNRIs).
Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Call your doctor right away if you have any of the following symptoms of serotonin syndrome:
- mental changes such as seeing things that are not there (hallucinations), agitation, or coma
- fast heartbeat
- changes in blood pressure
- high body temperature
- tight muscles
- trouble walking
- nausea, vomiting, or diarrhea
What are eletriptan hydrobromide tablets?
Eletriptan hydrobromide tablets are a prescription medicine used to treat acute migraine headaches with or without aura in adults.
Eletriptan hydrobromide tablets are for people who have been diagnosed with migraine headaches.
Eletriptan hydrobromide tablets are not used to prevent or decrease the number of migraine headaches you have.
It is not known if eletriptan hydrobromide tablets are safe and effective to treat cluster headaches.
It is not known if eletriptan hydrobromide tablets are safe and effective in children.
Who should not take eletriptan hydrobromide tablets?
Do not take eletriptan hydrobromide tablets if you:
- have heart problems or a history of heart problems
- have or have had a stroke or problems with your blood circulation
- have hemiplegic or basilar migraines. If you are not sure if you have these types of migraines, ask your doctor
- have narrowing of the blood vessels in your legs, arms, stomach, or kidney (peripheral vascular disease)
- have ischemic bowel disease
- have uncontrolled high blood pressure
- have taken any of the following medicines in the last 24 hours:
- other “triptans” or triptan combination products such as:
- almotriptan (Axert®)
- frovatriptan (Frova®)
- naratriptan (Amerge®)
- rizatriptan (Maxalt®)
- sumatriptan and naproxen sodium, (Treximet®)
- zolmitriptan (Zomig®)
- sumatriptan (Imitrex®)
- ergotamines such as:
- Bellergal-S®
- Cafergot®
- Ergomar®
- Wigraine®
- dihydroergotamines such as:
- D.H.E. 45® or Migranal® or methysergide (Sansert®)
- have taken the following medicines within the last 72 hours:
- ketoconazole (Nizoral®)
- itraconazole (Sporanox®)
- nefazodone (Serzone®)
- troleandomycin (TAO®)
- clarithromycin (Biaxin®)
- ritonavir (Norvir®)
- nelfinavir (Viracept®)
- are allergic to eletriptan or any of the ingredients in eletriptan hydrobromide tablets. See the end of this leaflet for a complete list of ingredients in eletriptan hydrobromide tablets.
What should I tell my doctor before taking eletriptan hydrobromide tablets?
Before you take eletriptan hydrobromide tablets, tell your doctor if you:
- have heart problems or family history of heart problems or stroke
- have high blood pressure
- have high cholesterol
- have diabetes
- smoke
- are overweight
- are a female who has gone through menopause
- have kidney problems
- have liver problems
- are pregnant or plan to become pregnant. It is not known if eletriptan hydrobromide will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Eletriptan hydrobromide passes into your breast milk and may harm your baby. Talk to your doctor about the best way to feed your baby if you take eletriptan hydrobromide tablets.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Your doctor will decide if you can take eletriptan hydrobromide tablets with your other medicines. Eletriptan hydrobromide tablets and other medicines may affect each other causing side effects.
Especially tell your doctor if you take anti-depressant medicines called:
- selective serotonin reuptake inhibitors (SSRIs)
- serotonin and norepinephrine reuptake inhibitors (SNRIs)
Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor or pharmacist when you get a new medicine.
How should I take eletriptan hydrobromide tablets?
- Take eletriptan hydrobromide tablets exactly as your doctor tells you to take it.
- Your doctor will tell you how much eletriptan hydrobromide tablets to take and when to take it.
- Your doctor may change your dose if needed. Do not change your dose without first talking to your doctor.
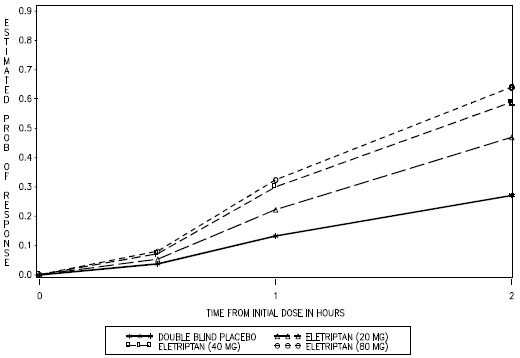
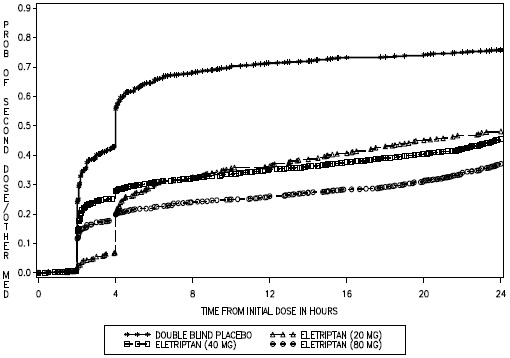
- Take 1 eletriptan hydrobromide tablet as soon as you feel a migraine coming on.
- If you do not get any relief after your first eletriptan hydrobromide tablet, do not take a second tablet without first talking with your doctor.
- If your headache comes back or you only get some relief from your headache, you can take a second tablet 2 hours after the first tablet.
- Do not take more than a total of 80 mg of eletriptan hydrobromide tablets in a 24-hour period.
- If you take too much eletriptan hydrobromide, call your doctor or go to the nearest hospital emergency room right away.
- You should write down when you have headaches and when you take eletriptan hydrobromide tablets so you can talk to your doctor about how well eletriptan hydrobromide tablets are working for you.
What should I avoid while taking eletriptan hydrobromide tablets?
Eletriptan hydrobromide tablets can cause dizziness, weakness, or drowsiness. If you have these symptoms, do not drive a car, use machinery, or do anything where you need to be alert.
What are the possible side effects of eletriptan hydrobromide tablets?
Eletriptan hydrobromide tablets may cause serious side effects. See “What is the most important information I should know about eletriptan hydrobromide tablets?”
These serious side effects include:
-
changes in color or sensation in your fingers and toes (Raynaud’s syndrome)
-
stomach and intestinal problems (gastrointestinal and colonic ischemic events). Symptoms of gastrointestinal and colonic ischemic events include:
- sudden or severe stomach pain
- stomach pain after meals
- weight loss
- nausea or vomiting
- constipation or diarrhea
- bloody diarrhea
- fever
-
problems with blood circulation to your legs and feet (peripheral vascular ischemia). Symptoms of peripheral vascular ischemia include:
- cramping and pain in your legs or hips
- feeling of heaviness or tightness in your leg muscles
- burning or aching pain in your feet or toes while resting
- numbness, tingling, or weakness in your legs
- cold feeling or color changes in 1 or both legs or feet
- medication overuse headaches. Some people who take too many eletriptan hydrobromide tablets may have worse headaches (medication overuse headache). If your headaches get worse, your doctor may decide to stop your treatment with eletriptan hydrobromide tablets.
The most common side effects of eletriptan hydrobromide tablets include:
- dizziness
- nausea
- weakness
- tiredness
- drowsiness
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of eletriptan hydrobromide tablets. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store eletriptan hydrobromide tablets?
- Store eletriptan hydrobromide tablets at room temperature between 20° to 25°C (68° to 77°F).
General information about the safe and effective use of eletriptan hydrobromide tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use eletriptan hydrobromide tablets for a condition for which it was not prescribed. Do not give eletriptan hydrobromide tablets to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information summarizes the most important information about eletriptan hydrobromide tablets. If you would like more information about eletriptan hydrobromide tablets, talk with your doctor. You can ask your doctor or pharmacist for information on eletriptan hydrobromide tablets that is written for health professionals.
For more information call Aurobindo Pharma USA, Inc. at 1-866-850-2876 or go to www.aurobindousa.com.
What are the ingredients in eletriptan hydrobromide tablets?
Active ingredient: eletriptan hydrobromide
Inactive ingredients: croscarmellose sodium, FD&C yellow 6 aluminum lake, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, titanium dioxide and triacetin.
The brands listed are the trademarks of their respective owners and are not trademarks of the Aurobindo Pharma Limited.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 038, India
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 04/2020
Close