Label: AMOXICILLIN AND CLAVULANATE POTASSIUM powder, for suspension
- NDC Code(s): 59651-025-01, 59651-025-55, 59651-025-75, 59651-026-01, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMOXICILLIN AND CLAVULANATE POTASSIUM FOR ORAL SUSPENSION safely and effectively. See full prescribing information for AMOXICILLIN ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAmoxicillin and clavulanate potassium for oral suspension is indicated for the treatment of infections in adults and pediatric patients, due to susceptible isolates of the designated bacteria in ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Amoxicillin and clavulanate potassium for oral suspension may be taken without regard to meals; however, absorption of clavulanate potassium is ...
-
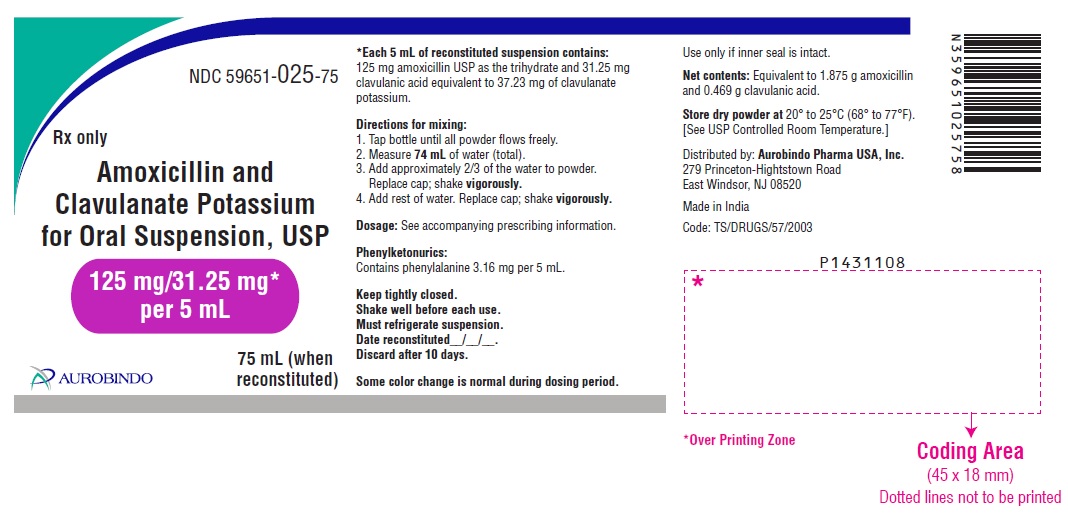
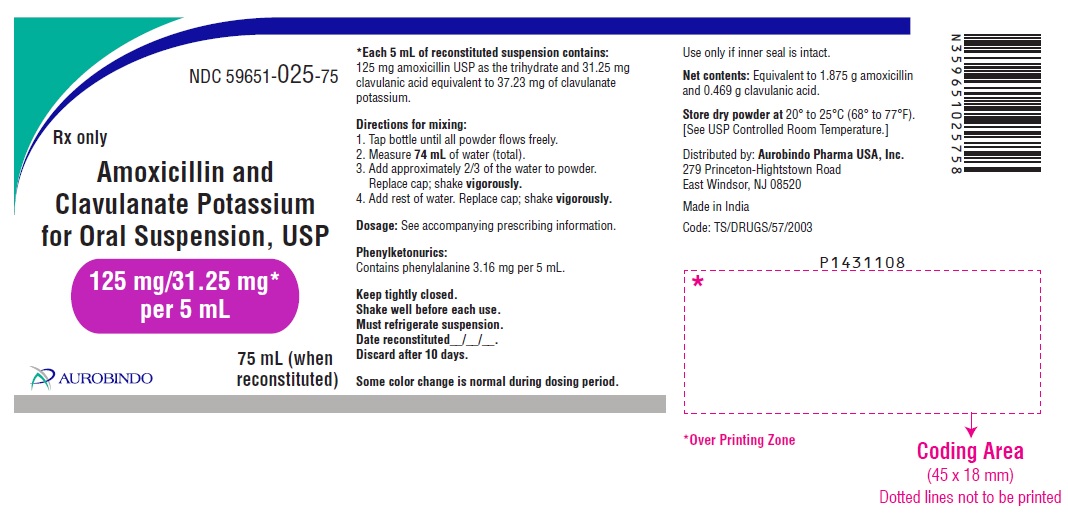
3 DOSAGE FORMS AND STRENGTHS125 mg/31.25 mg per 5 mL: White to off-white granular powder – Each 5 mL of reconstituted white to pale yellow, orange flavored suspension contains 125 mg of amoxicillin as the trihydrate ...
-
4 CONTRAINDICATIONS4.1 Serious Hypersensitivity Reactions - Amoxicillin and clavulanate potassium is contraindicated in patients with a history of serious hypersensitivity reactions (e.g., anaphylaxis or ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving beta-lactam antibacterials, including ...
-
6 ADVERSE REACTIONSThe following are discussed in more detail in other sections of the labeling: Anaphylactic reactions [see Warnings and Precautions (5.1)] Severe Cutaneous Adverse Reactions [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Probenecid - Probenecid decreases the renal tubular secretion of amoxicillin but does not delay renal excretion of clavulanic acid. Concurrent use with amoxicillin and clavulanate potassium ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects: Pregnancy Category B. Reproduction studies performed in pregnant rats and mice given amoxicillin and clavulanate potassium (2:1 ratio formulation of ...
-
10 OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures as required. A prospective study of 51 pediatric patients at a poison-control center ...
-
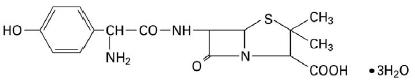
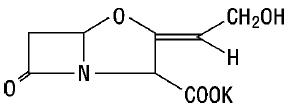
11 DESCRIPTIONAmoxicillin and clavulanate potassium for oral suspension, USP is an oral antibacterial combination consisting of amoxicillin and the beta-lactamase inhibitor, clavulanate potassium (the potassium ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Amoxicillin and clavulanate potassium is an antibacterial drug [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Mean amoxicillin and clavulanate potassium ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Amoxicillin and clavulanate potassium (4:1 ...
-
14 CLINICAL STUDIES14.1 Lower Respiratory Tract and Complicated Urinary Tract Infections - Data from 2 pivotal trials in 1,191 patients treated for either lower respiratory tract infections or complicated urinary ...
-
15 REFERENCESSwanson-Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol. 1988; 30: 66-67.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAmoxicillin and Clavulanate Potassium for Oral Suspension USP, 125 mg/31.25 mg per 5 mL is a white to off-white granular powder – Each 5 mL of reconstituted white to pale yellow, orange flavored ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions - Inform patients that amoxicillin and clavulanate potassium for oral suspension may be taken every 8 hours or every 12 hours, depending on the dose prescribed. Each ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 125 mg/31.25 mg per 5 mL (75 mL Bottle Label)NDC 59651-025-75 - Rx only - Amoxicillin and - Clavulanate Potassium - for Oral Suspension, USP - 125 mg/31.25 mg* per 5 mL - 75 mL (when - reconstituted) AUROBINDO
-
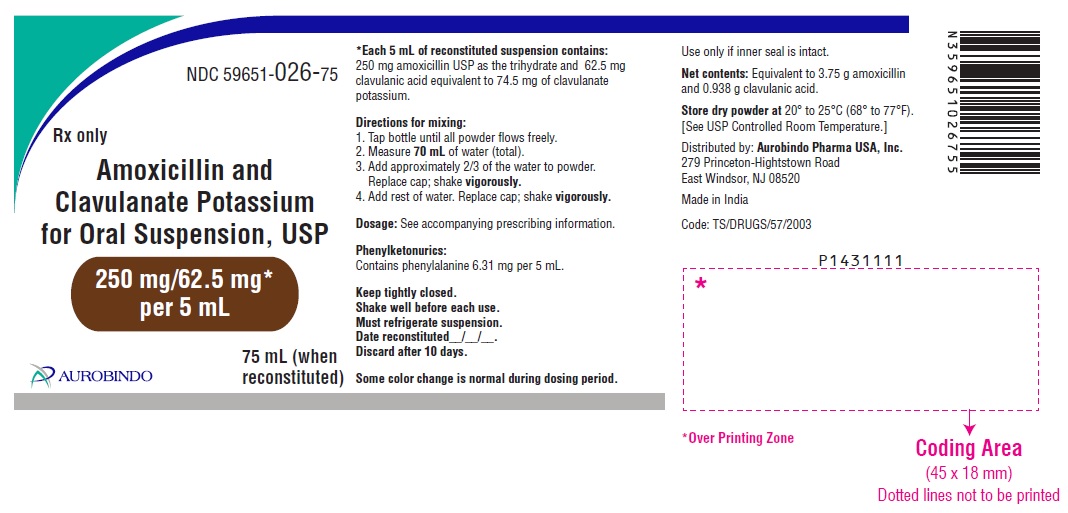
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg/62.5 mg per 5 mL (75 mL Bottle Label)NDC 59651-026-75 - Rx only - Amoxicillin and - Clavulanate Potassium - for Oral Suspension, USP - 250 mg/62.5 mg* per 5 mL - 75 mL (when - reconstituted) AUROBINDO
-
INGREDIENTS AND APPEARANCEProduct Information