Label: HALOPERIDOL tablet
- NDC Code(s): 59651-789-01, 59651-789-99, 59651-790-01, 59651-790-99, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Haloperidol is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
Close -
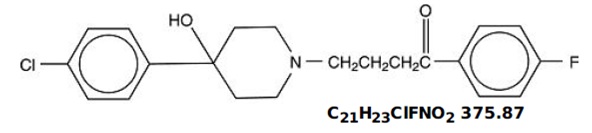
DESCRIPTIONHaloperidol is the first of the butyrophenone series of major tranquilizers. The chemical designation is 4-[4-(p-chloro-phenyl)-4-hydroxypiperidino]-4’—fluorobutyrophenone and it has the following ...
-
CLINICAL PHARMACOLOGYThe precise mechanism of action has not been clearly established.
-
INDICATIONS AND USAGEHaloperidol tablets are indicated for use in the management of manifestations of psychotic disorders. Haloperidol tablets are indicated for the control of tics and vocal utterances of Tourette’s ...
-
CONTRAINDICATIONSHaloperidol tablets are contraindicated in severe toxic central nervous system depression or comatose states from any cause and in individuals who are hypersensitive to this drug or have ...
-
WARNINGSIncreased Mortality in Elderly Patients with Dementia-Related Psychosis - Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death ...
-
PRECAUTIONSLeukopenia, Neutropenia and Agranulocytosis - In clinical trial and post-marketing experience, events of leukopenia/neutropenia have been reported temporally related to antipsychotic agents ...
-
ADVERSE REACTIONSCardiovascular Effects - Tachycardia, hypotension, and hypertension have been reported. QT prolongation and/or ventricular arrhythmias have also been reported, in addition to ECG pattern changes ...
-
OVERDOSAGEManifestations - In general, the symptoms of overdosage would be an exaggeration of known pharmacologic effects and adverse reactions, the most prominent of which would be: 1) severe ...
-
DOSAGE AND ADMINISTRATIONThere is considerable variation from patient to patient in the amount of medication required for treatment. As with all antipsychotic drugs, dosage should be individualized according to the needs ...
-
HOW SUPPLIEDHaloperidol Tablets, USP are available containing 0.5 mg, 1 mg, 2 mg, 5 mg, 10 mg or 20 mg of haloperidol USP. The 0.5 mg tablets are white to off-white, round uncoated tablets, debossed with ...
-
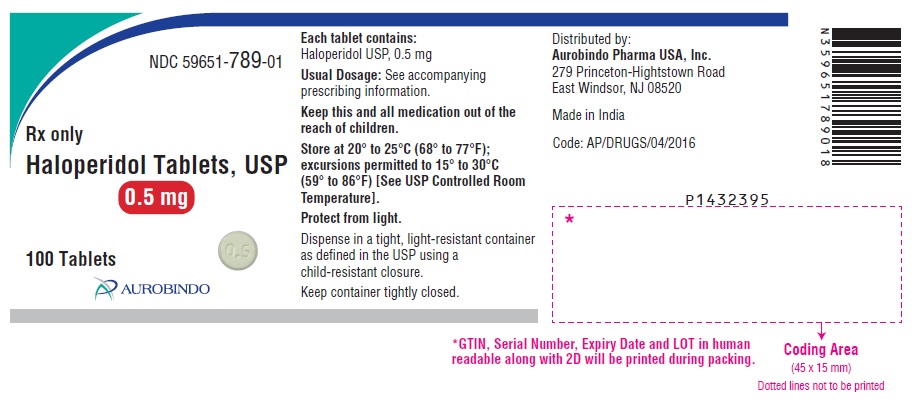
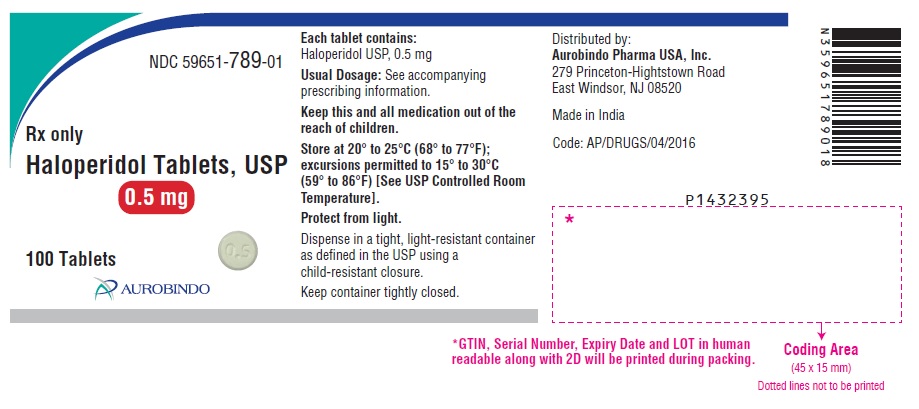
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 0.5 mg (100 Tablets Bottle)NDC 59651-789-01 - Rx only - Haloperidol Tablets, USP - 0.5 mg - 100 Tablets - AUROBINDO
-
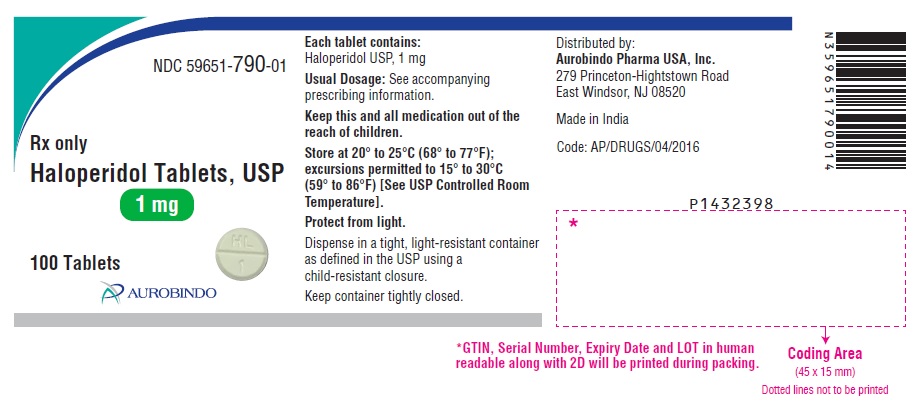
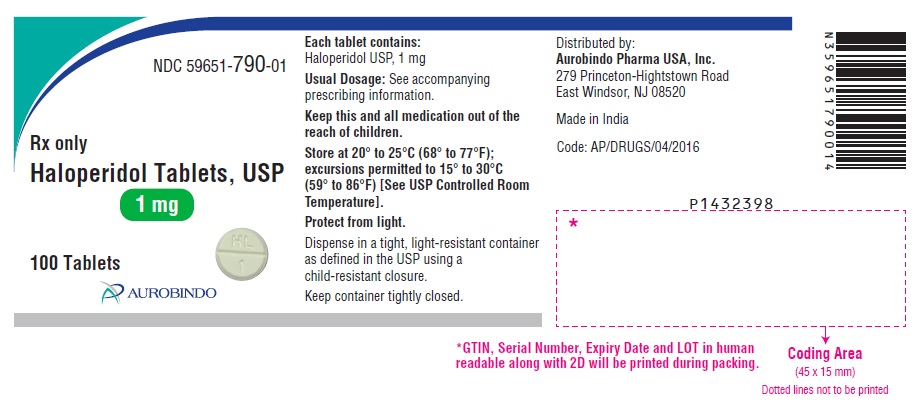
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 1 mg (100 Tablets Bottle)NDC 59651-790-01 - Rx only - Haloperidol Tablets, USP - 1 mg - 100 Tablets - AUROBINDO
-
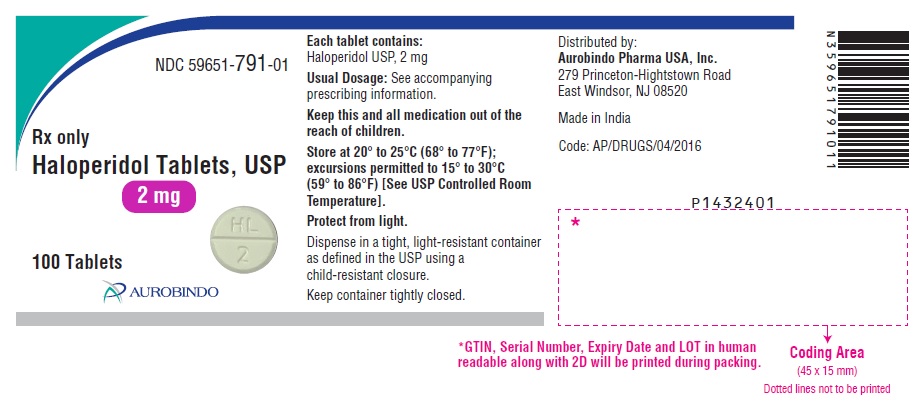
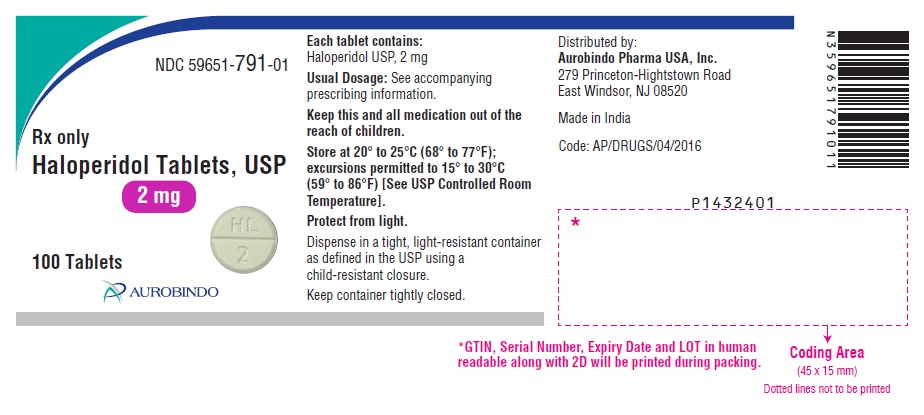
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 2 mg (100 Tablets Bottle)NDC 59651-791-01 - Rx only - Haloperidol Tablets, USP - 2 mg - 100 Tablets - AUROBINDO
-
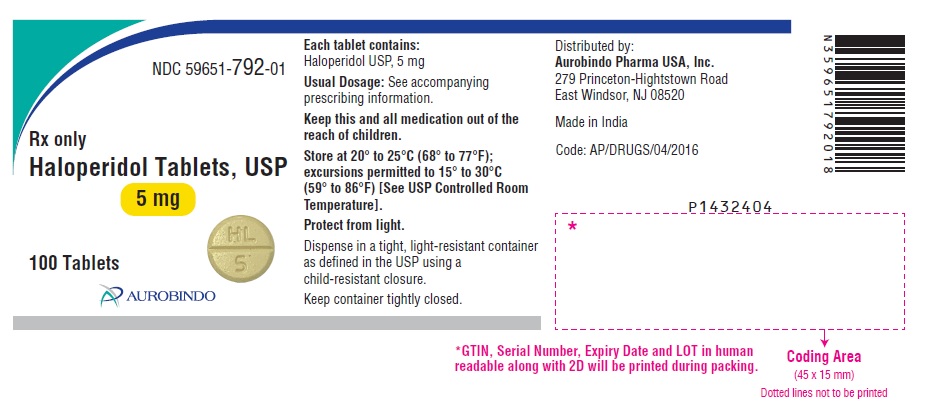
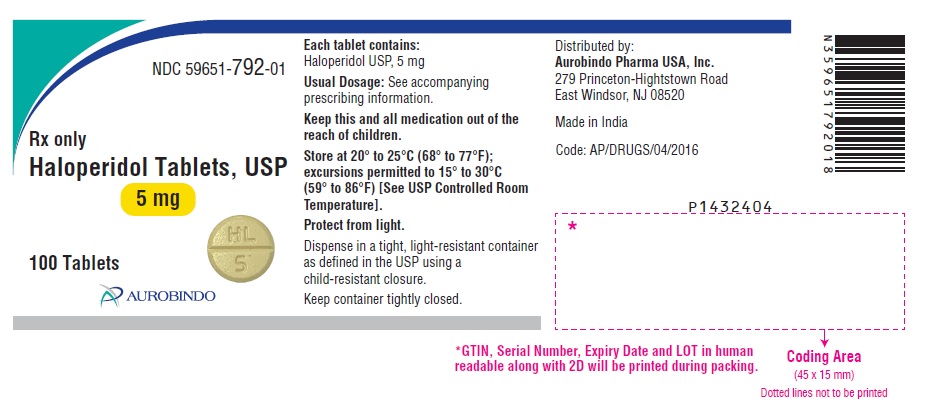
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 5 mg (100 Tablets Bottle)NDC 59651-792-01 - Rx only - Haloperidol Tablets, USP - 5 mg - 100 Tablets - AUROBINDO
-
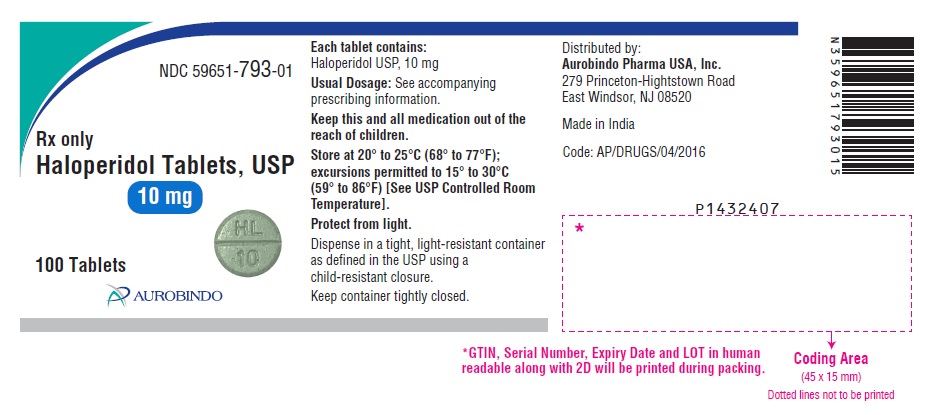
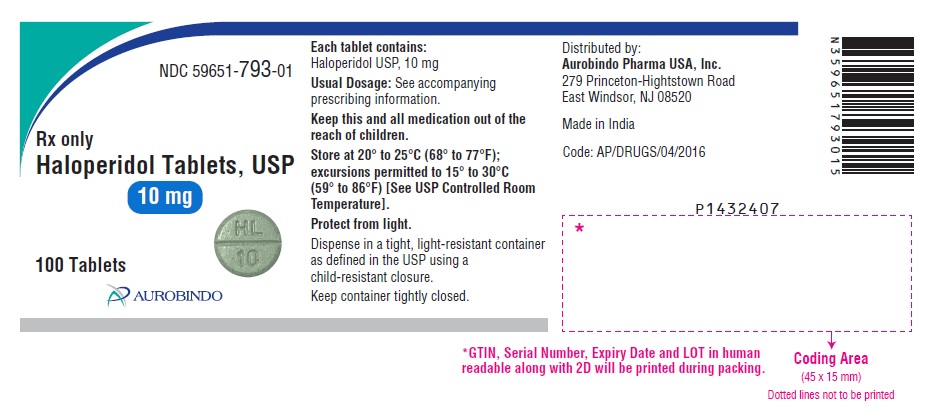
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 10 mg (100 Tablets Bottle)NDC 59651-793-01 - Rx only - Haloperidol Tablets, USP - 10 mg - 100 Tablets - AUROBINDO
-
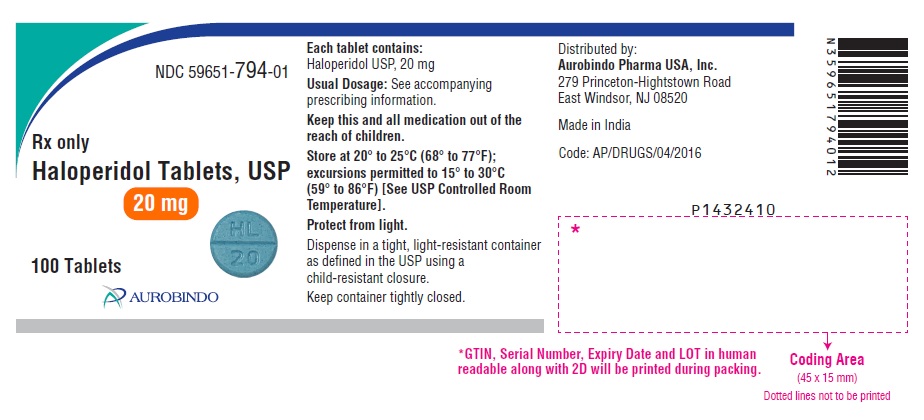
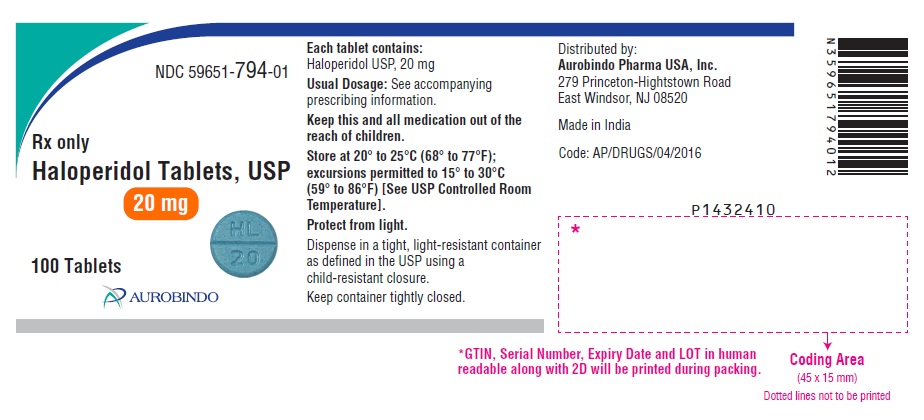
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 20 mg (100 Tablets Bottle)NDC 59651-794-01 - Rx only - Haloperidol Tablets, USP - 20 mg - 100 Tablets - AUROBINDO
-
INGREDIENTS AND APPEARANCEProduct Information