Label: HYDROCORTISONE tablet
- NDC Code(s): 59651-413-50, 59651-414-01, 59651-415-01
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
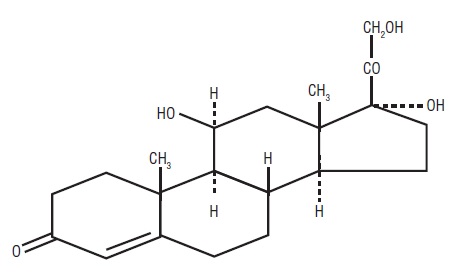
DESCRIPTIONHydrocortisone Tablets USP contain hydrocortisone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from ...
-
INDICATIONS AND USAGEHydrocortisone tablets are indicated in the following conditions. 1. Endocrine Disorders - Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice ...
-
CONTRAINDICATIONSSystemic fungal infections and known hypersensitivity to components
-
WARNINGSIn patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is ...
-
PRECAUTIONSGeneral Precautions - Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after ...
-
ADVERSE REACTIONSFluid and Electrolyte Disturbances - Sodium retention - Fluid retention - Congestive heart failure in susceptible patients - Potassium loss - Hypokalemic ...
-
DOSAGE AND ADMINISTRATIONThe initial dosage of hydrocortisone tablets may vary from 20 mg to 240 mg of hydrocortisone per day depending on the specific disease entity being treated. In situations of less severity lower ...
-
HOW SUPPLIEDHydrocortisone Tablets USP are available in the following strengths and package sizes: Hydrocortisone Tablets USP, 5 mg are white to off-white, round shaped tablets, with score line on one side ...
-
REFERENCES1Fekety R. Infections associated with corticosteroids and immunosuppressive therapy. In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious Diseases. Philadelphia: WB Saunders Company ...
-
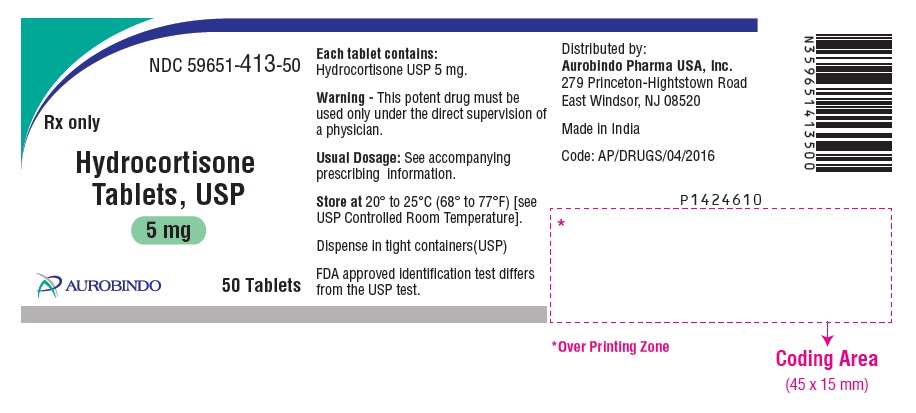
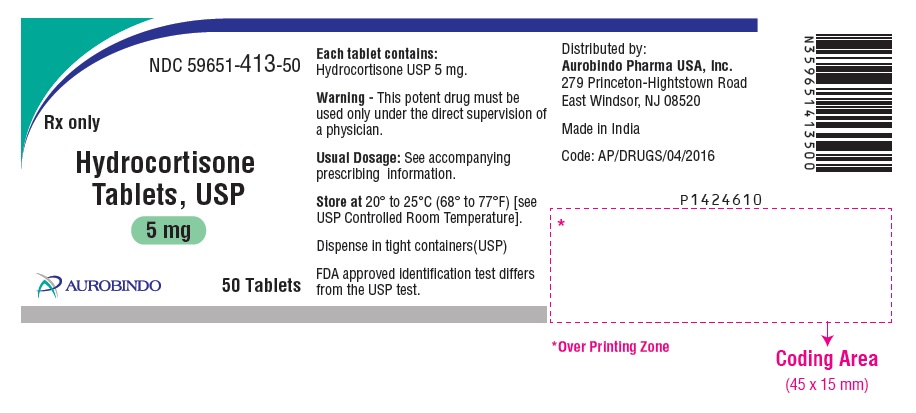
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg (50 Tablets Bottle)NDC 59651-413-50 - Rx only - Hydrocortisone - Tablets, USP - 5 mg - AUROBINDO 50 Tablets
-
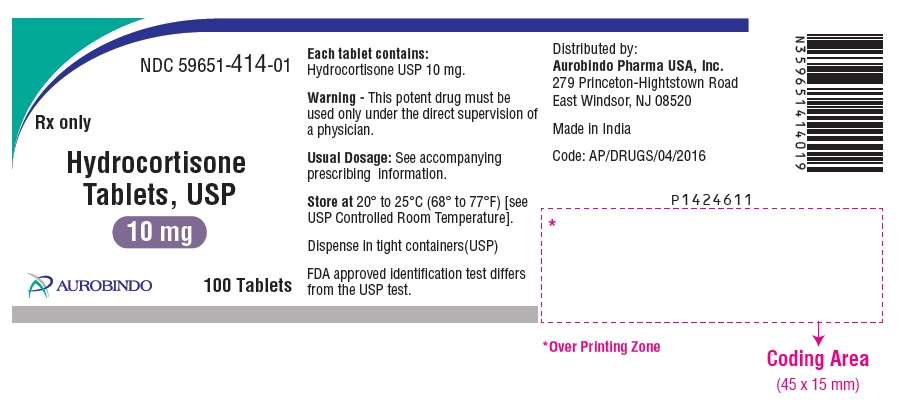
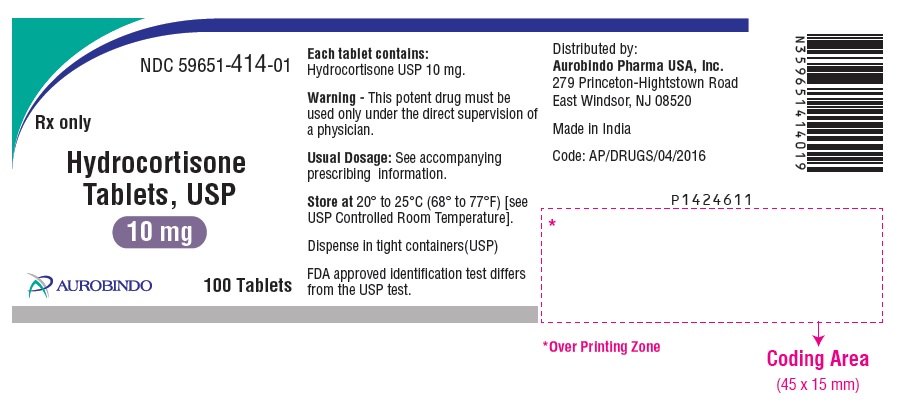
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (100 Tablets Bottle)NDC 59651-414-01 - Rx only - Hydrocortisone - Tablets, USP - 10 mg - AUROBINDO 100 Tablets
-
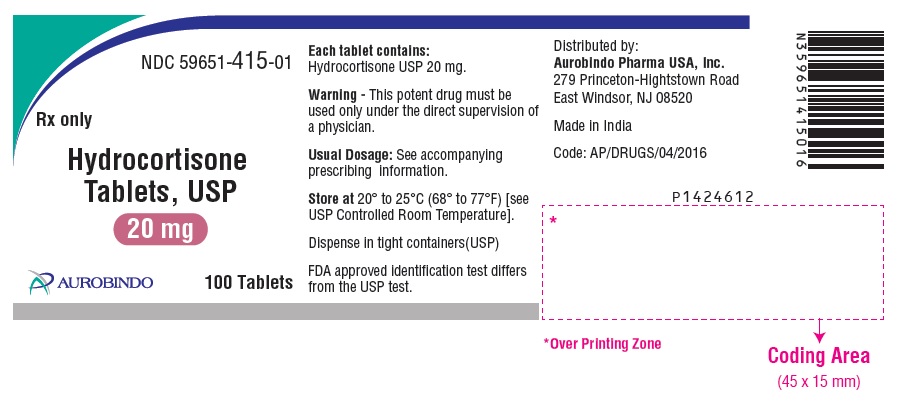
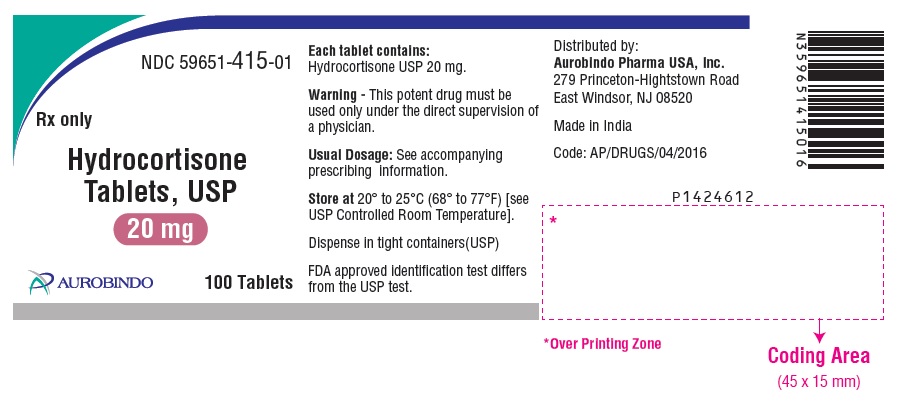
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (100 Tablets Bottle)NDC 59651-415-01 - Rx only - Hydrocortisone - Tablets, USP - 20 mg - AUROBINDO 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information