Label: IBUPROFEN IMMEDIATE RELEASE- ibuprofen tablet, coated
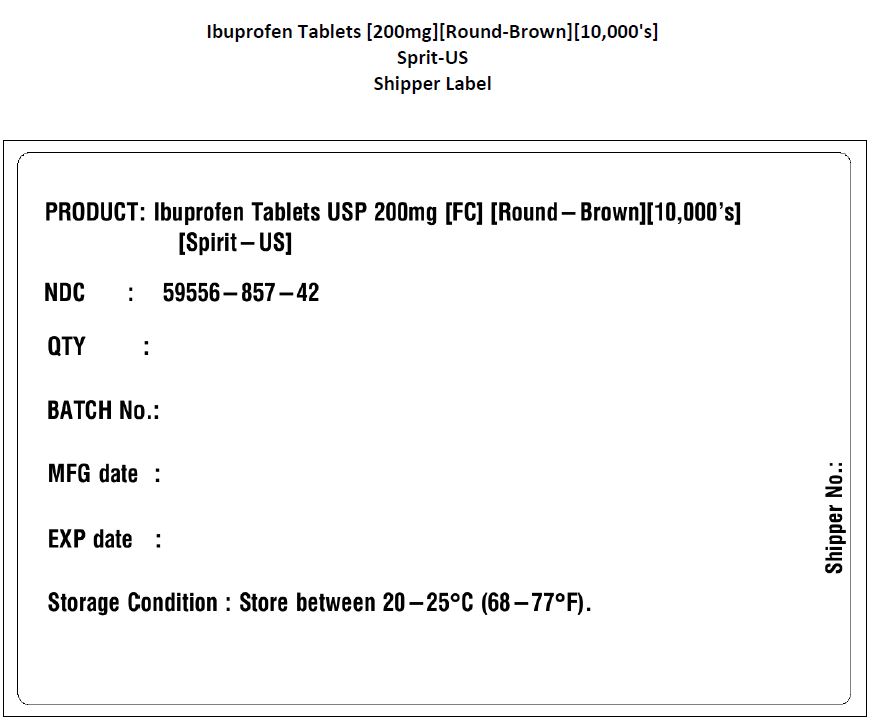
- NDC Code(s): 59556-857-42, 59556-858-42
- Packager: Strides Pharma Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- BOXED WARNING (What is this?)
-
ACTIVE INGREDIENTActive ingredient (in each Brown tablet or caplet**) Ibuprofen 200 mg (NSAID)* * nonsteroidal anti-inflammatory drug - **capsule-shaped tablets
-
PURPOSEPurpose - Pain reliever / fever reducer
-
Uses
temporarily relieves minor aches and pains due to: headache - muscular aches - minor pain of arthritis - toothache - backache - the common cold - menstrual cramps - temporarily reduces fever
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: hives - facial swelling asthma (wheezing) shock ...
-
Do not Useif you have ever had an allergic reaction to ibuprofen or any other pain reliever / fever reducer - right before or after heart surgery
-
ASK DOCTOR/PHARMACISTAsk a doctor before use if - you have problems or serious side effects from taking pain relievers or fever reducers - the stomach bleeding warning applies to you - you have a history of stomach ...
-
SPL UNCLASSIFIED SECTIONAsk a doctor or pharmacist before use if you are - taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin - under a doctor's care for any serious ...
-
WHEN USINGWhen using this product - take with food or milk if stomach upset occurs
-
STOP USEStop use and ask a doctor if - you experience any of the following signs of stomach bleeding: feel faint - vomit blood have bloody or black stools have ...
-
PREGNANCY OR BREAST FEEDINGIf pregnant or breast-feeding, ask a health professional before use. It is especially important not to use ibuprofen at 20 weeks or later in pregnancy unless definitely directed to do so by a ...
-
KEEP OUT OF REACH OF CHILDRENKeep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
-
SPL UNCLASSIFIED SECTIONDirections - do not take more than directed - the smallest effective dose should be used - adults and children 12 years and older - take 1 tablet every 4 to 6 hours while ...
-
STORAGE AND HANDLINGOther Information - store between 20-250C (68-770F) do not use if carton is opened. Do not use if foil inner seal imprinted with "Sealed For Your Safety" is broken or missing
-
Inactive Ingredients
colloidal silicon dioxide, corn starch, hypromellose, magnesium stearate, microcrystalline cellulose, pregelatinized starch, red iron oxide, sodium starch glycolate, talc, titanium dioxide and ...
-
Questions or Comments?Call 1-855-742-7868 (toll-free)
-
SPL UNCLASSIFIED SECTIONManufactured by: Strides Pharma Science Limited, Puducherry - 605 014, India. Distributed by: Strides Pharma Inc. East Brunswick, NJ 08816 - January 2022
- DOSAGE & ADMINISTRATION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
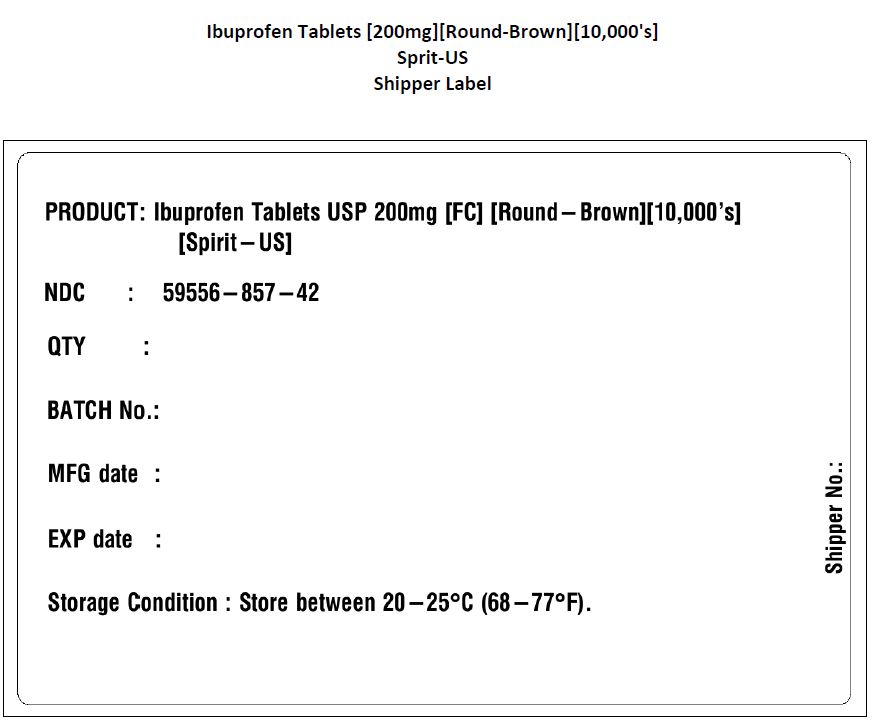 ...
... -
INGREDIENTS AND APPEARANCEProduct Information