Label: LIDOGEL- lidocaine hcl gel
- NDC Code(s): 59088-466-07
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
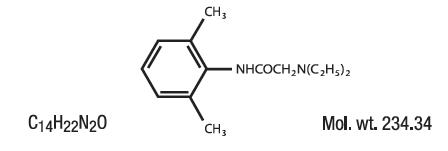
DESCRIPTIONContains Lidocaine HCl 2.8% in a mild acidic vehicle. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure: INGREDIENTS ...
-
CLINICAL PHARMACOLOGY:
MECHANISM OF ACTION: Lidogel™ 2.8% Gel releases Lidocaine from a mild acidic vehicle to stabilize the neuronal membrane by inhibiting the ionic fluxes - required for initiation and ...
-
CONTRAINDICATIONS:
Tuberculous or fungal lesions of skin vaccinia, varicella, and acute herpes simplex and in persons who have shown hypersensitivity to any of its components. Lidocaine is contraindicated in ...
-
WARNINGS:
For external use only. Not for ophthalmic use. Keep out of reach of children.
-
PRECAUTIONS:
If irritation of sensitivity occurs or infection appears, discontinue use and institute appropriate therapy. Lidogel™ 2.8% Gel should - be used with caution in ill, elderly, debilitated ...
-
CARCINOGENESIS, MUTAGENESIS, AND IMPAIRMENT OF FERTILITY:
Studies of Lidocaine in animals to evaluate the carcinogenic potential of the effect on fertility have not been conducted.
-
USE IN PREGNANCY
Teratogenic Effects; Pregnancy Category B. Reproduction studies have been performed for Lidocaine in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to ...
-
NURSING MOTHERS:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be - exercised when this drug is administered to a nursing mother ...
-
PEDIATRIC USE:
Dosage in pediatric patients would be reduced commensurate with age, body weight, and physical condition.
-
ADVERSE REACTIONS:
During or immediately after treatment, the skin at the site of treatment may develop erythema or edema or may be the locus of - abnormal sensation.
-
DOSAGE AND ADMINISTRATION:
Apply a thin film to the affected area two or three times daily or as directed by a physician.
-
HOW SUPPLIED:
Lidogel™ 2.8% Gel 3.5 oz. (100 g) tube - NDC 59088-466-07 - KEEP THIS AND ALL MEDICATIONS OUT OF REACH OF CHILDREN.
-
STORAGE AND HANDLING:
Store at 20°-25°C (68°-77° F) [see USP Controlled Room Temperature]. Protect from freezing.
-
Lidogel™
Manufactured in the USA by: PureTek Corporation - Panorama City, CA 91402 - For questions or information - call toll-free: 877-921-7873
-
INGREDIENTS AND APPEARANCEProduct Information