Label: LIDOCORT- lidocaine hcl - hydrocortisone acetate cream
- NDC Code(s): 59088-430-05
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Anti-Inflammatory Anesthetic for Relief of Hemorrhoid Pain, Swelling and Inflammation.
Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure: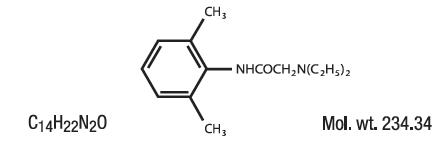
Hydrocortisone acetate has a chemical name pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11, 17-dihydroxy-(11β)-, and has the following structural formula: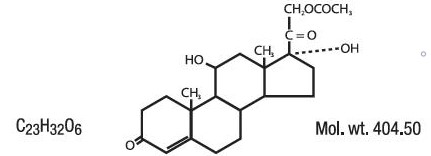
PharmaPure Rx Lidocaine HCl 3% - Hydrocortisone Acetate 0.5% Cream Each gram contains Lidocaine HCl 30 mg, Hydrocortisone Acetate 5 mg.
ACTIVE INGREDIENTS:
LIDOCAINE HCl 3%
HYDROCORTISONE ACETATE 0.5% - INACTIVE INGREDIENTS:
-
CLINICAL PHARMACOLOGY:
MECHANISM OF ACTION:
Product releases lidocaine to stabilize the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting local anesthetic action. Hydrocortisone acetate provides relief of inflammatory and pruritic manifestations of corticosteroid responsive dermatoses.
PHARMACOKINETICS:
Lidocaine may be absorbed following topical administration to mucous membranes, its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration, and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation of the liver.
Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjungation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2, 6-dimethylaniline.
The plasma binding of lidocaine is dependent of drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 g of free base per mL, 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid-glycoprotein.
Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
Studies of lidocaine metabolism following intravenous bolus injections have shown that the elimination half-life of this agent is typically 1.5 to 2 hours. Because of the rapid rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics but may increase the accumulation of metabolites.
Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6 g free base per mL. In the rhesus monkey arterial blood levels of 18-21 g/mL have been shown to be the threshold for convulsive activity.
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma protein in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile. - INDICATIONS:
-
CONTRAINDICATIONS:
Product should not be used in patients with a history of sensitivity to any of its ingredients or adverse reactions to lidocaine or amide anesthetics, which usually do not cross-react with “caine” ester type anesthetics. If excessive irritation and significant worsening occur, discontinue use and seek the advice of your physician. Product and topical lidocaine should be used cautiously in those with impaired liver function, as well as the very ill or very elderly and those with significant liver disease. Product should be used with caution in patients receiving antiarrhythmic drugs of Class I since the adverse effects are additive and generally synergistic. Product is contraindicated for tuberculous or fungal lesions of skin vaccinia, varicella and acute herpes simplex. Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any components of the preparation.
-
WARNINGS:
For external use only. Not for ophthalmic use. Product and used applicators could harm small children if chewed or swallowed.
Keep out of reach of children.
Topical formulations of lidocaine may be absorbed to a greater extent through mucous membranes and abraded, fissured or irritated skin than through intact skin. Product should not be ingested or applied into the mouth, inside of the nose or in the eyes. Product should not be used in the ears. Any situation where lidocaine penetrates beyond the tympanic membrane into the middle ear is contraindicted because of ototoxicty associated with lidocaine observed in animals when instilled in the middle ear. Product should not come into contact with the eye or be applied into the eye because of the risk of severe eye irritation and the loss of eye surface sensation, which reduces protective reflexes and can lead to corneal irritation and possibly abrasion. If eye contact occurs, rinse out the eye immediately with saline or water and protect the eye surface until sensation is restored. -
PRECAUTIONS:
If irritation or sensitivity occurs or infection appears, discontinue use and institute appropriate therapy. If extensive areas are treated, the possibility of systemic absorption exists. Systemic absorption of topical steroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestation of Cushing’s syndrome, hyperglycemia, and glycosuria in some patients. Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings. Therefore, patients receiving a large dose of potent topical steroids applied to a large surface area, or under an occlusive dressing, should be evaluated periodically for evidence of HPA axis suppression. If noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Recovery of the HPA axis function is generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids. Children may absorb proportionately larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity. If irritation develops, topical steroids should be discontinued and appropriate therapy instituted. In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
-
CARCINOGENESIS, MUTAGENESIS, AND IMPAIRMENT OF FERTILITY:
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids.
Studies to determine mutagenicity with prednisolone and hydrocortisone have revealed negative results.
Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential of the effect on fertility have not been conducted. -
USE IN PREGNANCY:
Teratogenic Effects:
Pregnancy Category C Reproduction studies have been performed for lidocaine in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering lidocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place. Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts or for prolonged periods of time.
- NURSING MOTHERS:
- PEDIATRIC USE:
- ADVERSE REACTIONS:
-
DOSAGE AND ADMINISTRATION:
Apply product to the affected area(s) twice daily or as directed by a physician. Product should not be used in excess of recommendations or for prolonged used in the anal canal. If the condition does not respond to repeated courses of product or should worsen, discontinue use and seek the advise of your physician.
Products without applicators: Remove the child-resistant cap and foil seal from the tube. Apply a thin film to the affected area. Replace the cap after use.Products with Single-Use Tubes and Applicators: Tear open one cleansing wipe packet (if the product kit contains such item), gently clean the affected area and discard the used cleansing wipe. Remove the child-resistant cap and foil seal from one tube and firmly screw one applicator onto the tube. Do not over tighten. Squeeze the tube to fill the applicator until a small amount of cream/gel comes out of and lubricates the applicator openings. Gently insert the applicator tip with attached tube into anal area. Continue squeezing the body of the tube as it is moved around the areas of discomfort, and lastly, around and in the anal opening (if directed by physician).
Do not completely insert the applicator and tube into the anus or insert deep into the rectum. Do not insert a loose applicator tip into the anus or rectum. Once application is completed, both the tube and applicator should be gently removed and discarded.
- HOW SUPPLIED:
- KEEP THIS AND ALL MEDICATIONS OUT OF REACH OF CHILDREN.
- Lidocort: LIDOCAINE HCl 3% HYDROCORTISONE ACETATE 0.5%
-
INGREDIENTS AND APPEARANCE
LIDOCORT
lidocaine hcl - hydrocortisone acetate creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59088-430 Route of Administration TOPICAL, RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 30 mg in 1 g HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 5 mg in 1 g Inactive Ingredients Ingredient Name Strength STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CETYL ALCOHOL (UNII: 936JST6JCN) METHYLPARABEN (UNII: A2I8C7HI9T) PROPANEDIOL (UNII: 5965N8W85T) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) STEARIC ACID (UNII: 4ELV7Z65AP) CALCIUM ACETATE (UNII: Y882YXF34X) MINERAL OIL (UNII: T5L8T28FGP) POLYSORBATE 60 (UNII: CAL22UVI4M) ALUMINUM SULFATE (UNII: 34S289N54E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-430-05 85 g in 1 TUBE; Type 0: Not a Combination Product 09/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/01/2020 Labeler - PureTek Corporation (785961046) Establishment Name Address ID/FEI Business Operations PureTek Corporation 785961046 manufacture(59088-430) , label(59088-430)


