Label: HYDROXYM- hydrocortisone gel
- NDC Code(s): 59088-209-03
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
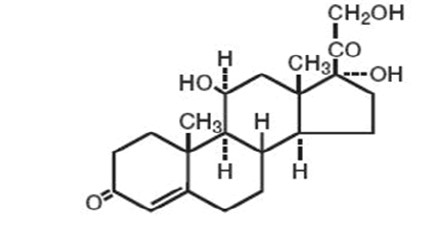
DESCRIPTIONEach gram of Hydroxym Gel contains 20 mg of hydrocortisone. Inactive ingredients include: Aloe Barbadensis (Aloe Vera) Leaf Juice, Aqua (Purified Water), Hydroxyethyl Cellulose, Methylparaben ...
-
CLINICAL PHARMACOLOGYMechanism of Action- Topical corticosteroids share anti-inflammatory, antipruritic, and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the topical corticosteroids is ...
-
INDICATIONS AND USAGETopical corticosteroids are indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatosis.
-
CONTRAINDICATIONSTopical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
-
WARNINGS:For external use only. Not for ophthalmic use. PRECAUTIONS - General - Systemic absorption of topical corticosteroids has produced reversible hypothalamicpituitary- adrenal (HPA) axis ...
-
ADVERSE REACTIONSThe following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an ...
-
OVERDOSAGETopically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (SSee PRECAUTIONS ).
-
DOSAGE AND ADMINISTRATIONTopical corticosteroids are generally applied to the affected area as a thin film from two to four times daily depending on the severity of the condition. Occlusive dressings may be used for the ...
-
HOW SUPPLIED:Hydroxym™ Gel is available as follows: 1 oz. (28 g) tube (NDC 59088-209-03) Do not use if tube seal is broken. KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. Store ...
-
Hydroxym™ Gel
Manufactured in the USA by: PureTek Corporation - Panorama City, CA 91402 - For questions or information - call toll-free: 877-921-7873
-
INGREDIENTS AND APPEARANCEProduct Information