Label: PYRIDOSTIGMINE BROMIDE tablet
- NDC Code(s): 58657-810-10, 58657-810-21, 58657-810-30
- Packager: Method Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONPYRIDOSTIGMINE BROMIDE TABLETS - Method Pharmaceuticals, LLC - ---------- PYRIDOSTIGMINE BROMIDE TABLETS
-
DESCRIPTION
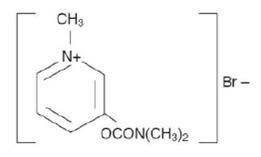
Pyridostigmine bromide tablets, USP - (pyridostigmine bromide) are an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide ...
-
CLINICAL PHARMACOLOGY
Pyridostigmine bromide tablets inhibits the destruction of acetylcholine by cholinesterase and thereby permits freer transmission of nerve impulses across the neuromuscular junction ...
-
INDICATIONS AND USAGE
Pyridostigmine bromide tablets are useful in the treatment of myasthenia gravis.
-
CONTRAINDICATIONS
Pyridostigmine bromide tablets are contraindicated in mechanical intestinal or urinary obstruction, and particular caution should be used in its administration to patients with bronchial asthma ...
-
WARNINGS
Although failure of patients to show clinical improvement may reflect under dosage, it can also be indicative of overdosage. As is true of all cholinergic drugs, overdosage of pyridostigmine ...
-
PRECAUTIONS
Pyridostigmine is mainly excreted unchanged by the kidney. 6,7,8 Therefore, lower doses may be required in patients with renal disease, and treatment should be based on titration of ...
-
ADVERSE REACTIONS
The side effects of pyridostigmine bromide tablets are most commonly related to overdosage and generally are of two varieties, muscarinic and nicotinic. Among those in the former group are nausea ...
-
DOSAGE AND ADMINISTRATION
Pyridostigmine bromide tablets, USP are available in following dosage form: Conventional Tablets - each containing 30 mg pyridostigmine bromide. Dosage - The size and frequency of the ...
-
HOW SUPPLIED
Tablets, are available as white, flat-faced tablets containing 30 mg pyridostigmine bromide, USP in bottles of 21 (NDC 58657-810-21) and cartons of 30 (3 x 10 blister packs - NDC ...
-
REFERENCES
Osserman KE, Genkins G. Studies in myasthenia gravis: Reduction in mortality rate after crisis. JAMA. Jan 1963; 183:97-101. Osserman KE, Genkins G. Studies in myasthenia gravis ...
-
PRINCIPAL DISPLAY PANEL
Unit-Of-Use - NDC 58657-810-21 - Pyridostigmine - Bromide Tablets, USP - 30 mg - Rx Only - 21 Tablets
-
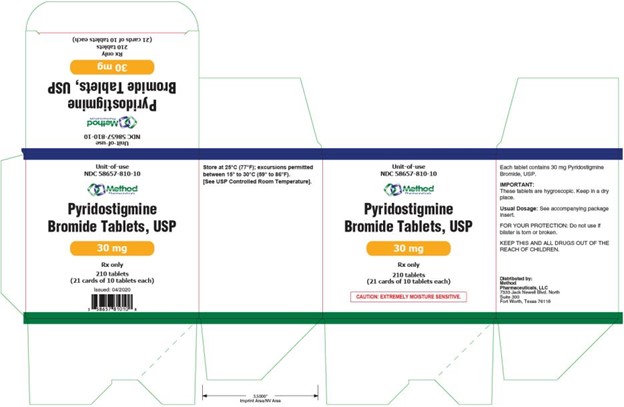
PRINCIPAL DISPLAY PANEL
Unit-Of-Use - NDC 58657-810-10 - Pyridostigmine - Bromide Tablets, USP - 30 mg - Rx Only - 210 Tablets - (21 cards of 10 tablets each)
-
INGREDIENTS AND APPEARANCEProduct Information