Label: NITROSTAT- nitroglycerin tablet
- NDC Code(s): 58151-309-01, 58151-310-01, 58151-310-52, 58151-310-94, view more
- Packager: Viatris Specialty LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NITROSTAT safely and effectively. See full prescribing information for NITROSTAT. NITROSTAT (nitroglycerin) sublingual ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE NITROSTAT is indicated for the acute relief of an attack or acute prophylaxis of angina pectoris due to coronary artery disease.
-
2 DOSAGE AND ADMINISTRATION Administer one tablet under the tongue or in the buccal pouch at the first sign of an acute anginal attack. Allow tablet to dissolve without swallowing. One additional tablet may be administered ...
-
3 DOSAGE FORMS AND STRENGTHS NITROSTAT is supplied as white, round, flat-faced tablets in three strengths: 0.3 mg (Coded with “N” on one side and “3” on the other) 0.4 mg (Coded with “N” on one side and “4” on the other) 0.6 ...
-
4 CONTRAINDICATIONS 4.1 PDE-5-Inhibitors and sGC-Stimulators - Do not use NITROSTAT in patients who are taking PDE-5 Inhibitors, such as avanafil, sildenafil, tadalafil, vardenafil hydrochloride. Concomitant use ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Tolerance - Excessive use may lead to the development of tolerance. Only the smallest dose required for effective relief of the acute angina attack should be used. A decrease in therapeutic ...
-
6 ADVERSE REACTIONS The following adverse reactions are discussed in more detail elsewhere in the label: • Hypotension [see Warnings and Precautions (5.2)] • Headache [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS 7.1 PDE-5-Inhibitors and sGC-Stimulators - NITROSTAT is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Limited published data on the use of nitroglycerin are insufficient to determine a drug associated risk of major birth defects or miscarriage. In animal ...
-
10 OVERDOSAGE 10.1 Signs and Symptoms, Methemoglobinemia - Nitrate overdosage may result in: severe hypotension, persistent throbbing headache, vertigo, palpitation, visual disturbance, flushing and ...
-
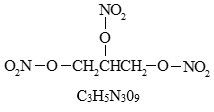
11 DESCRIPTION NITROSTAT is a stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg , or 0.6 mg nitroglycerin; as well as lactose monohydrate, NF; glyceryl monostearate, NF ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Nitroglycerin forms free radical nitric oxide (NO) which activates guanylate cyclase, resulting in an increase of guanosine 3'5' monophosphate (cyclic GMP) in smooth ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal carcinogenesis studies with sublingually administered nitroglycerin have not been performed. Carcinogenicity potential of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING NITROSTAT is supplied as white, round, flat-faced tablets in 3 strengths (0.3 mg, 0.4 mg, and 0.6 mg) in bottles containing 100 tablets each, with color-coded labels, and in color-coded Patient ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Distributed by: Viatris Specialty LLC - Morgantown, WV 26505 U.S.A. © 2023 Viatris Inc. NITROSTAT is a ...
-
Nitrostat®
(Nitroglycerin Sublingual Tablets, USP) Read this information carefully before you start NITROSTAT® (NYE-troe-stat) and each time you refill your prescription. There may be new information. This ...
-
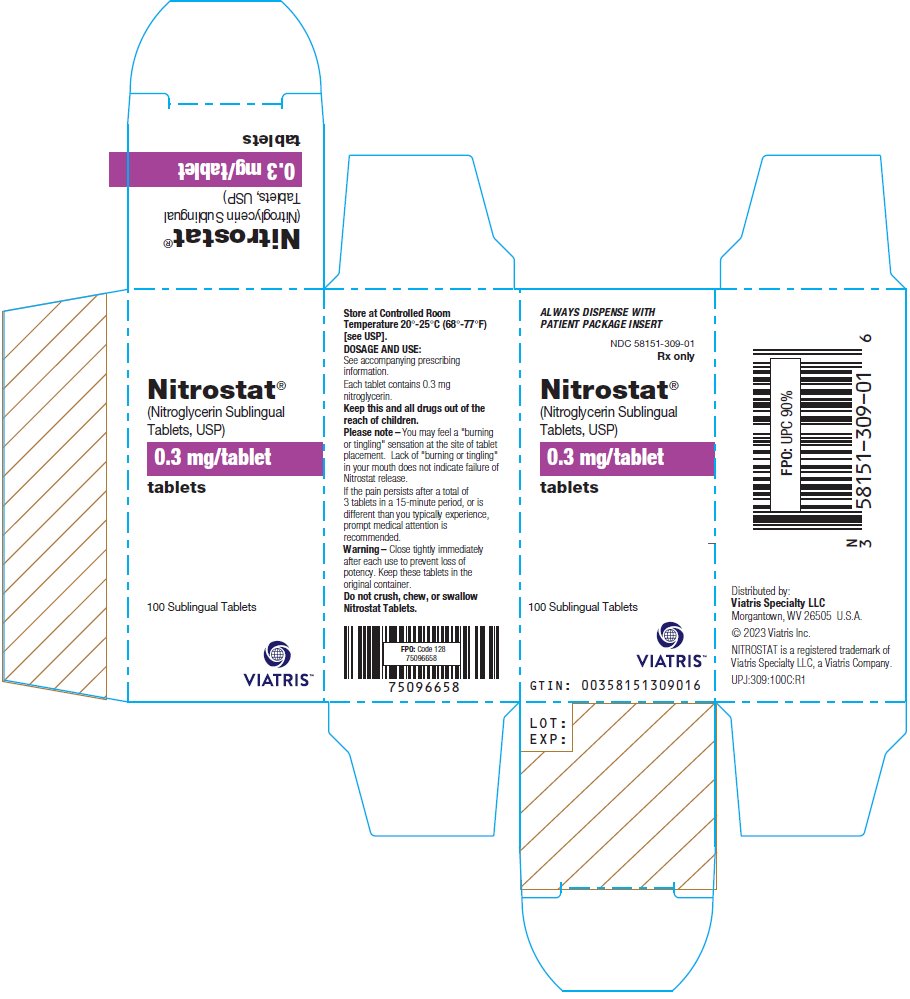
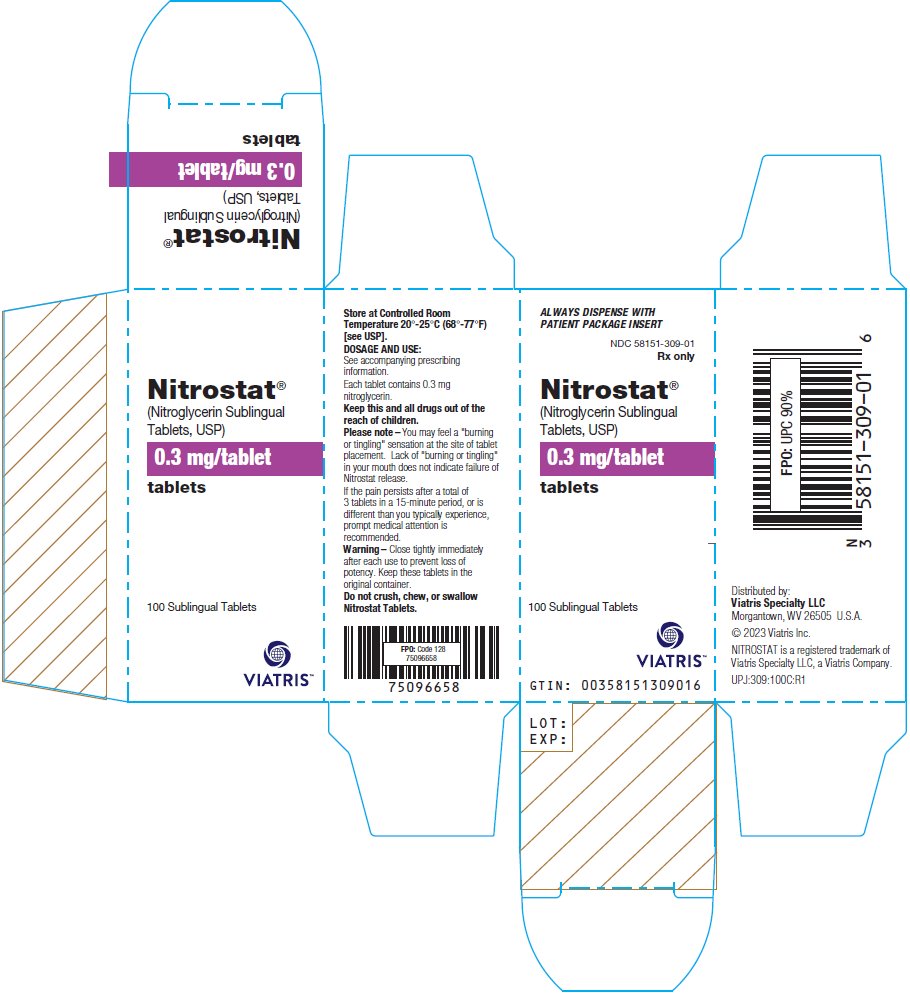
PRINCIPAL DISPLAY PANEL – 0.3 mg ALWAYS DISPENSE WITH - PATIENT PACKAGE INSERT - NDC 58151-309-01 - Rx only - Nitrostat® (Nitroglycerin Sublingual - Tablets, USP) 0.3 mg/tablet - tablets - 100 Sublingual Tablets - Store at Controlled ...
-
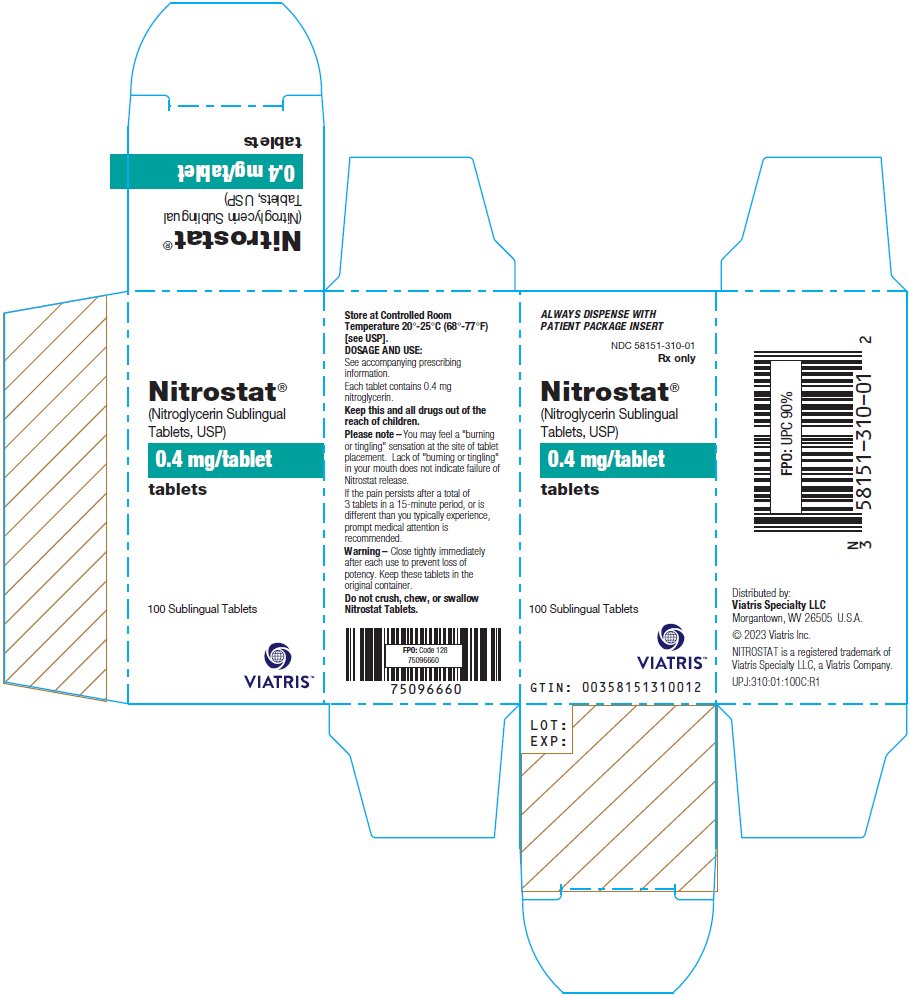
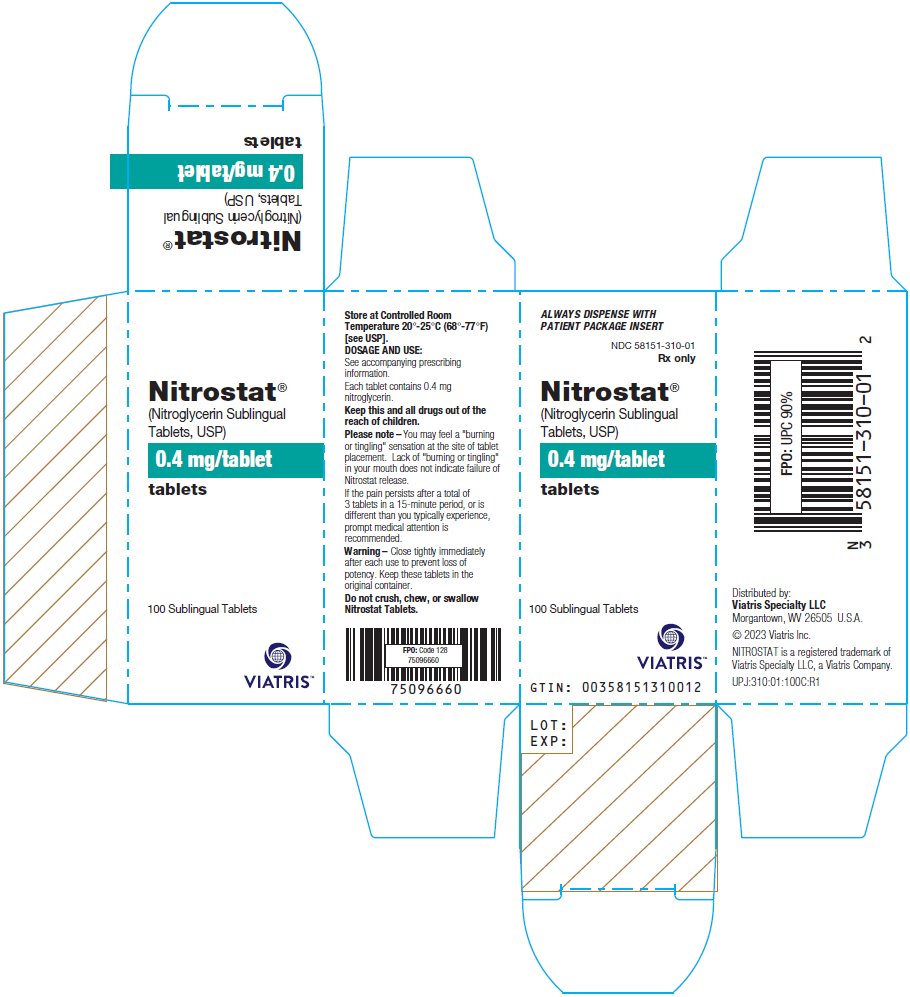
PRINCIPAL DISPLAY PANEL – 0.4 mg ALWAYS DISPENSE WITH - PATIENT PACKAGE INSERT - NDC 58151-310-01 - Rx only - Nitrostat® (Nitroglycerin Sublingual - Tablets, USP) 0.4 mg/tablet - tablets - 100 Sublingual Tablets - Store at Controlled ...
-
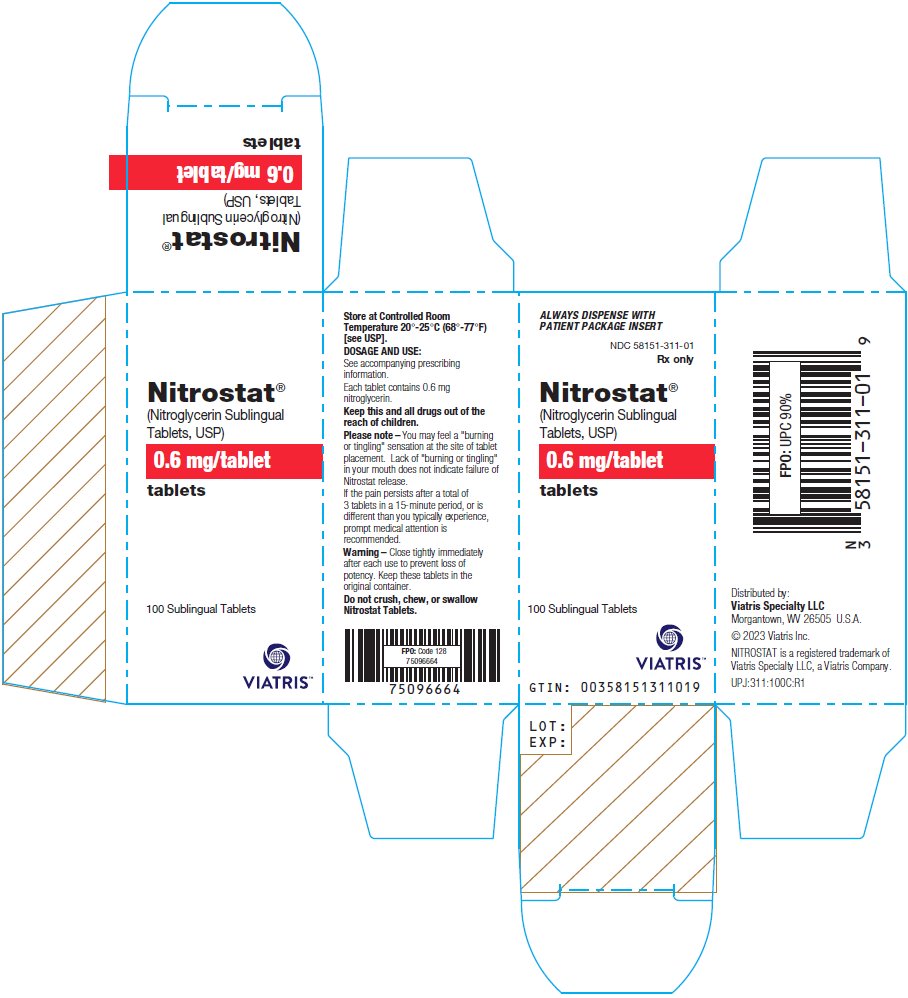
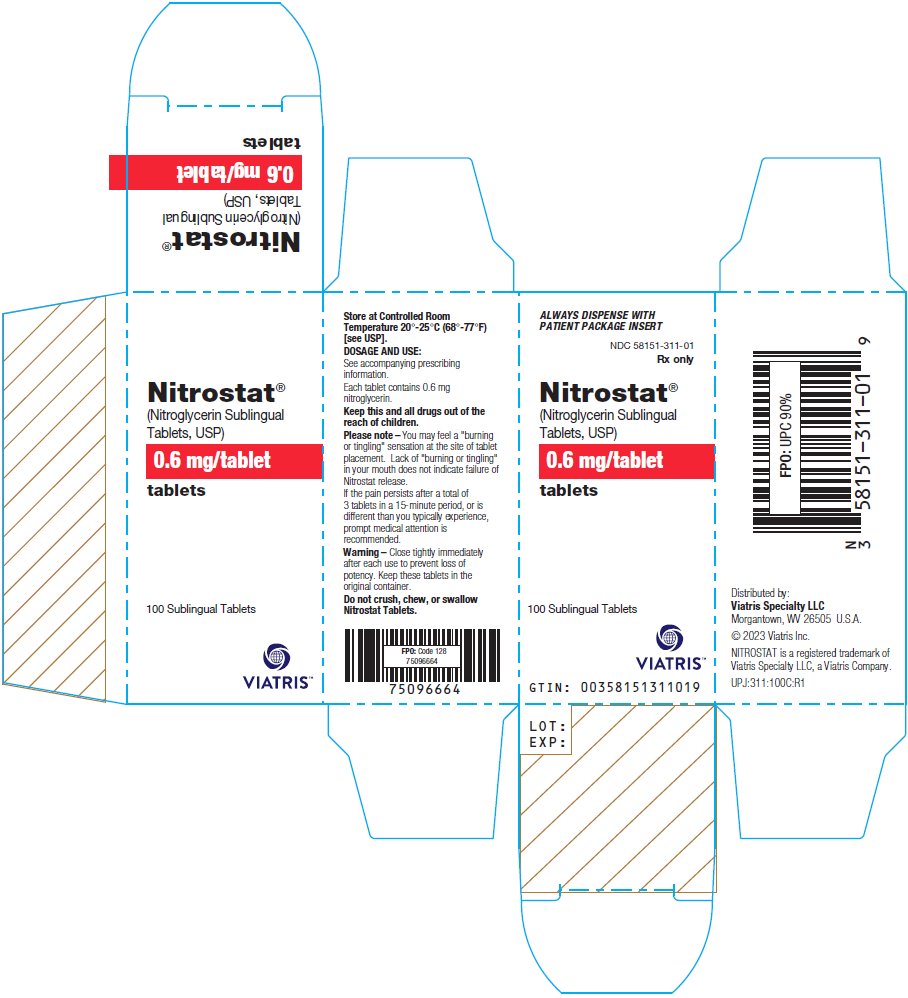
PRINCIPAL DISPLAY PANEL – 0.6 mg ALWAYS DISPENSE WITH - PATIENT PACKAGE INSERT - NDC 58151-311-01 - Rx only - Nitrostat® (Nitroglycerin Sublingual - Tablets, USP) 0.6 mg/tablet - tablets - 100 Sublingual Tablets - Store at Controlled ...
-
INGREDIENTS AND APPEARANCEProduct Information