Label: HYDROXYCHLOROQUINE SULFATE tablet
- NDC Code(s): 57664-761-13, 57664-761-88
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 11, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use HYDROXYCHLOROQUINE SULFATE TABLETS safely and effectively. See full prescribing information for HYDROXYCHLOROQUINE SULFATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Malaria - Hydroxychloroquine sulfate tablets are indicated in adult and pediatric patients for the: • Treatment of uncomplicated malaria due to Plasmodium falciparum, Plasmodium malariae ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Administer hydroxychloroquine sulfate tablets orally with food or milk. Do not crush or divide the tablets. 2.2 Dosage for Malaria in Adult and ...
-
3 DOSAGE FORMS AND STRENGTHSTablets, USP: 200 mg of hydroxychloroquine sulfate (equivalent to 155 mg base), white, to off-white, circular biconvex film coated tablets debossed with “347” on one side.
-
4 CONTRAINDICATIONSHydroxychloroquine sulfate tablets are contraindicated in patients with known hypersensitivity to 4-aminoquinoline compounds.
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiomyopathy and Ventricular Arrhythmias - Fatal and life-threatening cases of cardiotoxicity, including cardiomyopathy, have been reported in patients treated with hydroxychloroquine ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in greater detail in other sections: • Cardiomyopathy and Ventricular Arrhythmias [see Warnings and Precautions (5.1)] • Retinal Toxicity [see ...
-
7 DRUG INTERACTIONS7.1 Drugs Prolonging QT Interval and Other Arrhythmogenic Drugs - Hydroxychloroquine sulfate tablets prolong the QT interval. There may be an increased risk of inducing ventricular arrhythmias if ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to hydroxychloroquine sulfate tablets during pregnancy ...
-
10 OVERDOSAGEHydroxychloroquine sulfate tablets overdosage symptoms have an onset within 1–3 hours of ingestion. The following have been reported with hydroxychloroquine sulfate tablets ...
-
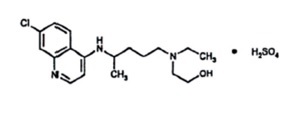
11 DESCRIPTIONHydroxychloroquine sulfate, USP is an antimalarial and antirheumatic drug, chemically described as 2-[[4-[(7-Chloro-4- quinolyl) amino]pentyl] ethylamino] ethanol sulfate (1:1) with the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Malaria - Hydroxychloroquine is a 4-aminoquinoline antimalarial [see Microbiology (12.4)] and antirheumatic agent. Rheumatoid Arthritis, Systemic Lupus Erythematosus ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or genotoxicity studies have been conducted with hydroxychloroquine. No animal studies have been performed to ...
-
15 REFERENCES1 Center for Disease Control and Prevention. Malaria. https://www.cdc.gov/parasites/malaria/index.html
-
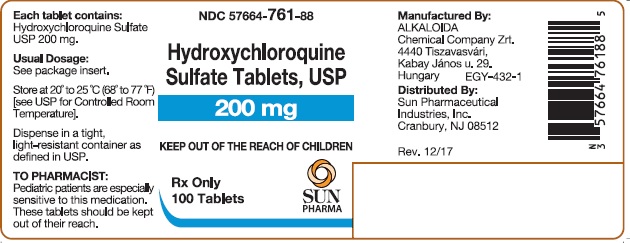
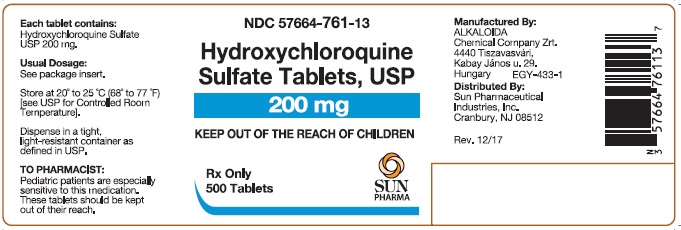
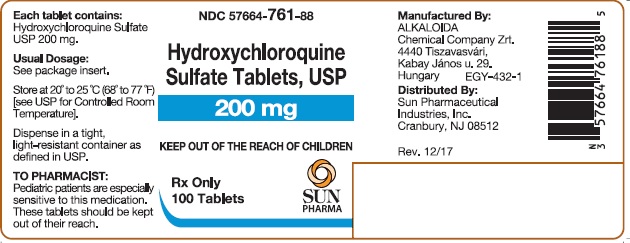
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Hydroxychloroquine sulfate tablets, USP contain 200 mg hydroxychloroquine sulfate, USP (equivalent to 155 mg base). Hydroxychloroquine sulfate tablets are white, to ...
-
17 PATIENT COUNSELING INFORMATIONImportant Administration Instructions - Advise the patient to take hydroxychloroquine sulfate tablets with food or milk and not to crush or divide the tablet. Cardiomyopathy and Ventricular ...
-
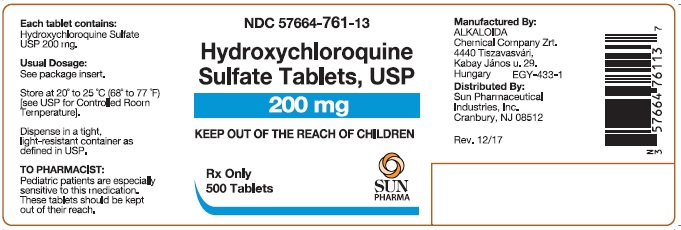
PACKAGE/LABEL PRINCIPAL DISPLAY PANELHydroxychloroquine Sulfate Tablets USP 200 mg; 100 Tablets - Hydroxychloroquine Sulfate Tablets USP 200 mg; 500 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information