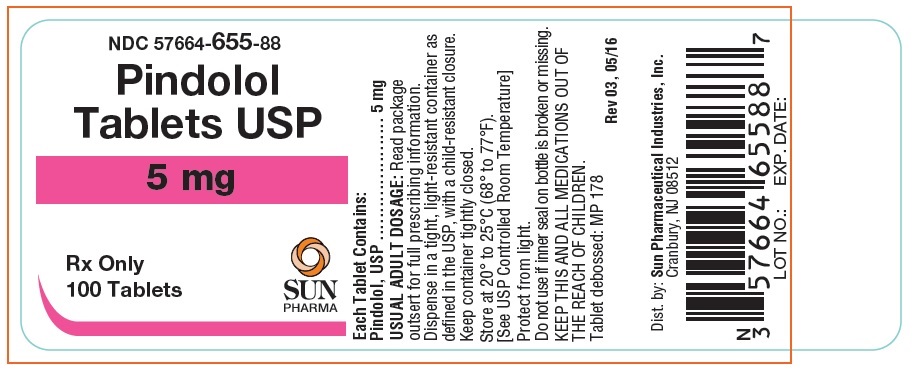
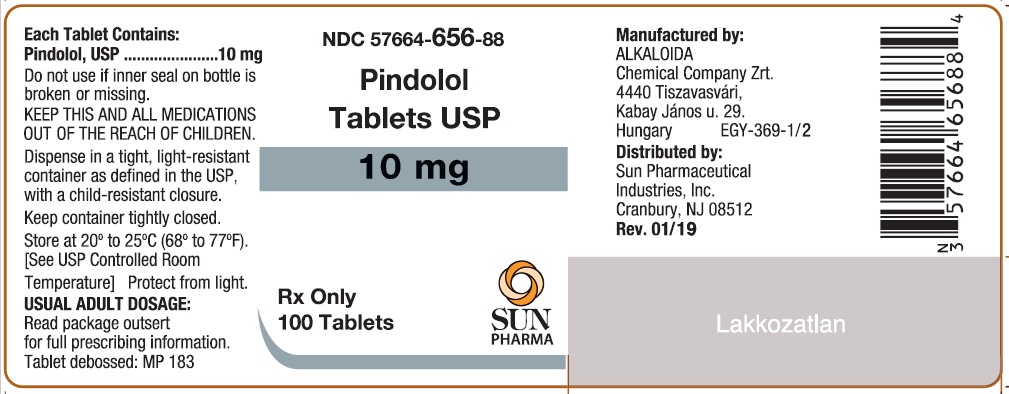
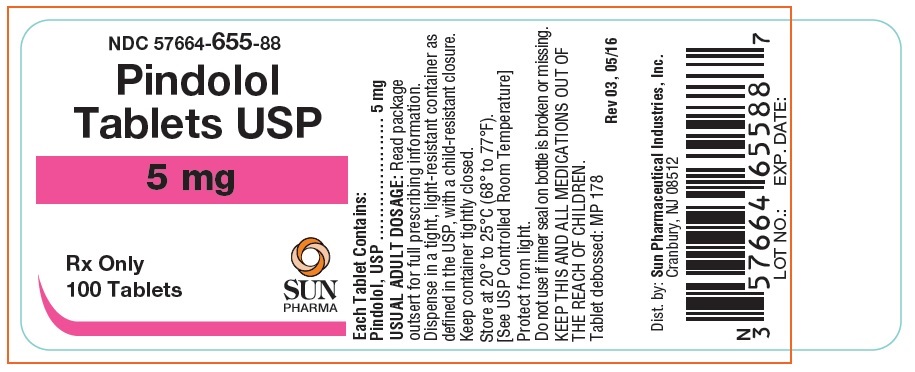
Label: PINDOLOL tablet
- NDC Code(s): 57664-655-88, 57664-656-88
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 10, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
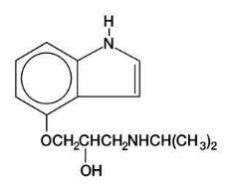
DESCRIPTIONPindolol, a synthetic beta-adrenergic receptor blocking agent with intrinsic sympathomimetic activity is 1-(Indol-4-yloxy)-3-(isopropylamino)-2-propanol. C14H20N2O2 M.W. 248.32 - Pindolol, USP ...
-
CLINICAL PHARMACOLOGYPindolol is a nonselective beta-adrenergic antagonist (beta-blocker) which possesses intrinsic sympathomimetic activity (ISA) in therapeutic dosage ranges but does not possess quinidine-like ...
-
INDICATIONS AND USAGEPindolol tablets are indicated in the management of hypertension. It may be used alone or concomitantly with other antihypertensive agents, particularly with a thiazide-type diuretic.
-
CONTRAINDICATIONSPindolol tablets are contraindicated in: 1) bronchial asthma; 2) overt cardiac failure; 3) cardiogenic shock; 4) second and third degree heart block; 5) severe bradycardia. (See WARNINGS.)
-
WARNINGSCardiac Failure - Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by beta-blockade may precipitate ...
-
PRECAUTIONSImpaired Renal or Hepatic Function - Beta-blocking agents should be used with caution in patients with impaired hepatic or renal function. Poor renal function has only minor effects on pindolol ...
-
CLINICAL LABORATORYMinor persistent elevations in serum transaminases (SGOT, SGPT) have been noted in 7% of patients during pindolol administration, but progressive elevations were not observed. These elevations ...
-
ADVERSE REACTIONSMost adverse reactions have been mild. The incidences listed in the following table are derived from 12-week comparative double-blind, parallel design trials in hypertensive patients given ...
-
POTENTIAL ADVERSE EFFECTSIn addition, other adverse effects not aforementioned have been reported with other beta-adrenergic blocking agents and should be considered potential adverse effects of pindolol. Central ...
-
OVERDOSAGENo specific information on emergency treatment of overdosage is available. Therefore, on the basis of the pharmacologic actions of pindolol, the following general measures should be employed as ...
-
DOSAGE AND ADMINISTRATIONThe dosage of pindolol tablets should be individualized. The recommended initial dose of pindolol tablets is 5 mg b.i.d. alone or in combination with other antihypertensive agents. An ...
-
HOW SUPPLIEDPindolol Tablets, USP are available as 5 mg and 10 mg tablets. The 5 mg tablets are round white tablets, debossed "MP 178" bisected on one side and blank on the other side. Bottles of 100 ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Sun Pharmaceutical Industries, Inc. Cranbury, NJ 08512 - Rev 02, September 2014
-
Pindolol Tablets USP 5mg, 100 Bottle Label (Frontida)
-
Pindolol Tablets USP 5mg, 100 Tablets Bottle label (Alkaloida)

-
Pindolol Tablets USP 10mg, 100 Tablets (Frontida)

-
Pindolol Tablets USP 10mg, 100 Tablets (Alkaloida)
-
INGREDIENTS AND APPEARANCEProduct Information