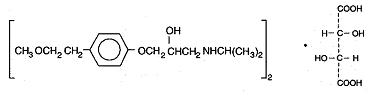Label: METOPROLOL TARTRATE tablet, film coated
- NDC Code(s): 57237-100-01, 57237-100-99, 57237-101-01, 57237-101-99, view more
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METOPROLOL TARTRATE TABLETS safely and effectively. See full prescribing information for METOPROLOL TARTRATE TABLETS. METOPROLOL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Hypertension - Metoprolol tartrate tablets are indicated for the treatment of hypertension in adult patients, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and ...
-
2 DOSAGE AND ADMINISTRATION2.1 Hypertension - Individualize the dosage of metoprolol tartrate tablets. Metoprolol tartrate tablets should be taken with or immediately following meals. The usual initial dosage is 100 mg ...
-
3 DOSAGE FORMS AND STRENGTHSMetoprolol tartrate tablets, USP are supplied as: 25 mg tablet – white round shaped, film coated tablets debossed with ‘C over 73’ on one side and deep break line on other side. 50 mg tablet ...
-
4 CONTRAINDICATIONSMetoprolol tartrate tablets are contraindicated in severe bradycardia, second- or third-degree heart block, cardiogenic shock, systolic blood pressure <100, decompensated heart failure, sick sinus ...
-
5 WARNINGS AND PRECAUTIONS5.1 Abrupt Cessation of Therapy - Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in labeling: Worsening angina or myocardial infarction [see Warnings and Precautions (5)] Worsening heart failure [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Catecholamine Depleting Drugs - Catecholamine depleting drugs (e.g., reserpine, monoamine oxidase (MAO) inhibitors) may have an additive effect when given with beta-blocking agents. Observe ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published observational studies have not demonstrated an association of adverse developmental outcomes with maternal use of metoprolol during ...
-
10 OVERDOSAGESigns and Symptoms - Overdosage of metoprolol may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include: atrioventricular block, heart failure ...
-
11 DESCRIPTIONMetoprolol tartrate tablets, USP contain metoprolol tartrate, a selective beta1-adrenoreceptor blocking agent. Metoprolol tartrate is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metoprolol is a beta1-selective (cardioselective) adrenergic receptor blocking agent. This preferential effect is not absolute, however, and at higher plasma ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in ...
-
14 CLINICAL STUDIES14.1 Hypertension - In controlled clinical studies, metoprolol has been shown to be an effective antihypertensive agent when used alone or as concomitant therapy with thiazide-type diuretics, at ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMetoprolol Tartrate Tablets, USP are available as follows: Tablets 25 mg are white round shaped, film coated tablets debossed with ‘C over 73’ on one side and deep break line on other side ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to take metoprolol tartrate tablets regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient should take only the ...
-
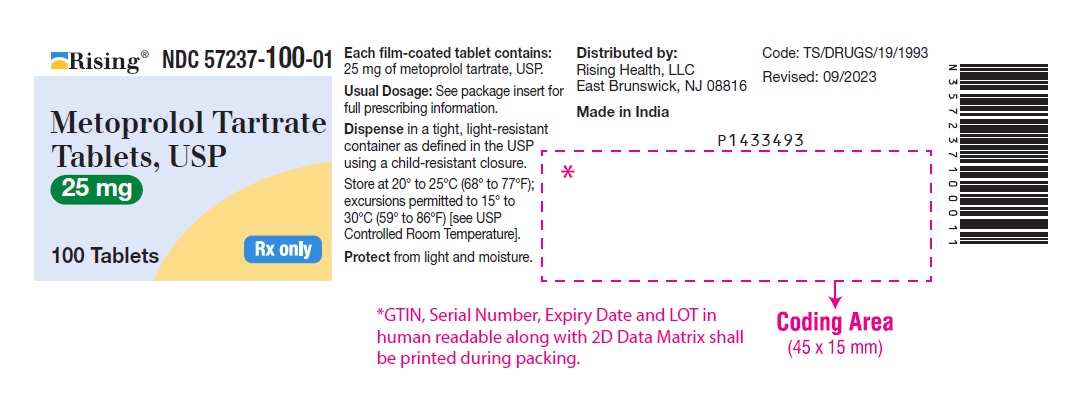
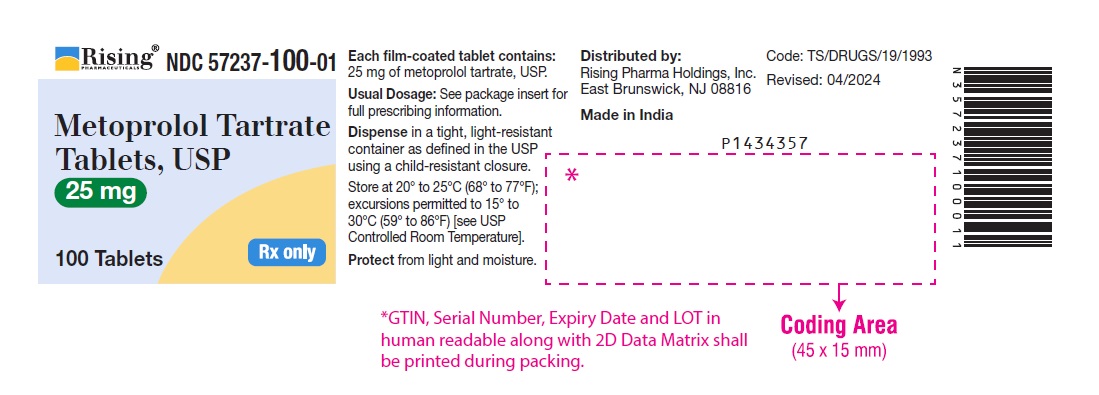
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 25 mg (100 Tablet Bottle)NDC 57237-100-01 - Metoprolol Tartrate - Tablets, USP - 25 mg - 100 Tablets Rx only
-
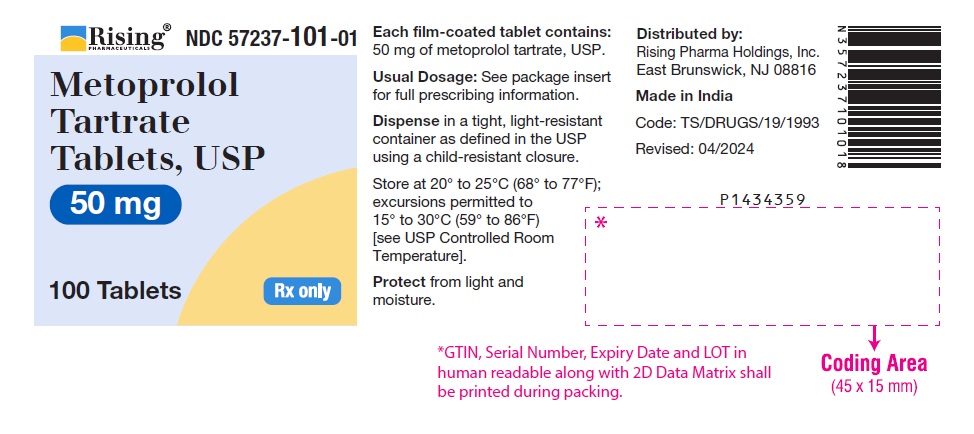
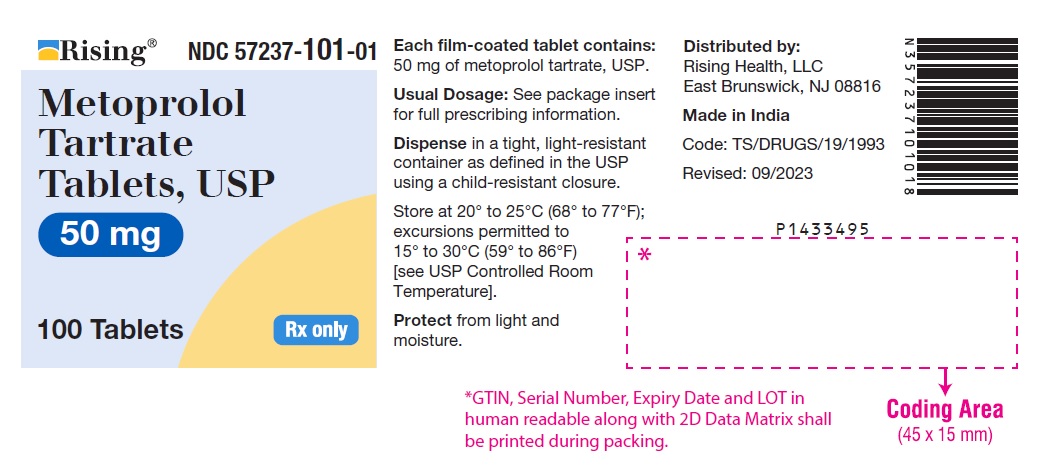
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 mg (100 Tablet Bottle)NDC 57237-101-01 - Metoprolol Tartrate - Tablets, USP - 50 mg - 100 Tablets Rx only
-
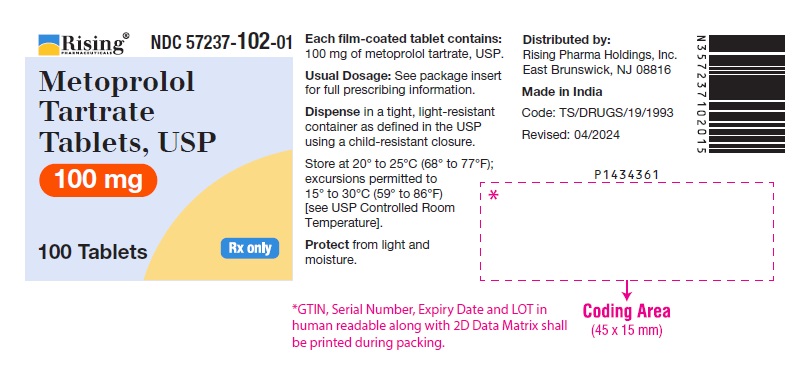
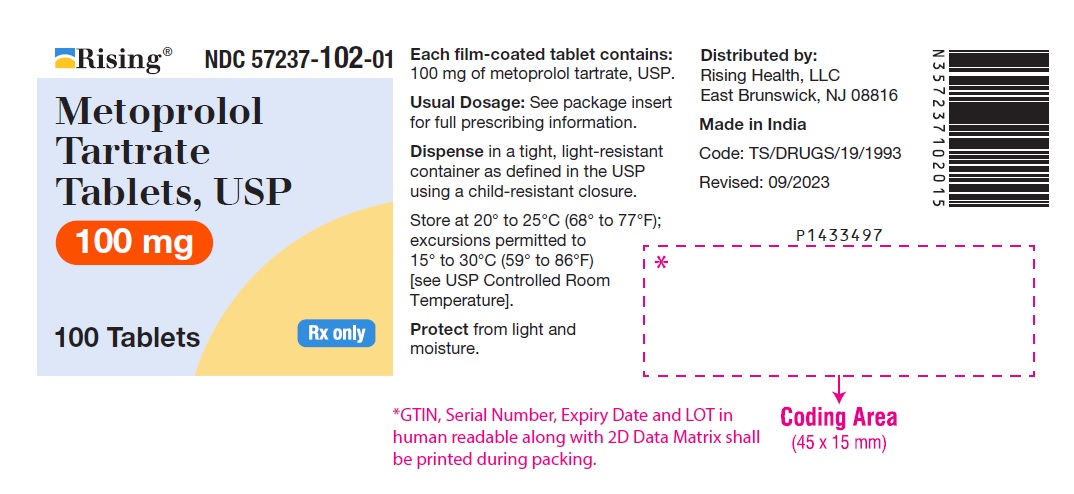
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 100 mg (100 Tablet Bottle)NDC 57237-102-01 - Metoprolol Tartrate - Tablets, USP - 100 mg - 100 Tablets Rx only
-
INGREDIENTS AND APPEARANCEProduct Information