Label: MINOCYCLINE HYDROCHLORIDE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 55111-637-01, 55111-637-10, 55111-638-01, 55111-638-10, view more - Packager: Dr. Reddy's Laboratories Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 9, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of minocycline hydrochloride tablets and other antibacterial drugs, minocycline hydrochloride tablets ...
-
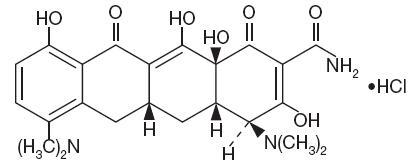
DESCRIPTIONMinocycline hydrochloride, USP a semisynthetic derivative of tetracycline, is 4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide ...
-
CLINICAL PHARMACOLOGYFollowing a single dose of one 100 mg tablet of minocycline hydrochloride administered to 28 normal fasting adult volunteers, maximum serum concentrations were attained in 1 to 3 hours (average ...
-
INDICATIONS AND USAGEMinocycline hydrochloride tablets are indicated in the treatment of the following infections due to susceptible strains of the designated microorganisms: Rocky Mountain spotted fever, typhus ...
-
CONTRAINDICATIONSThis drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines or to any of the components of the product formulation.
-
WARNINGSMINOCYCLINE HYDROCHLORIDE TABLETS, LIKE OTHER TETRACYCLINES-CLASS ANTIBIOTICS, CAN CAUSE FETAL HARM WHEN ADMINISTERED TO A PREGNANT WOMAN. IF ANY TETRACYCLINE IS USED DURING PREGNANCY OR IF THE ...
-
PRECAUTIONSMinocycline Hydrochloride Tablets, 50 mg contains FD&C Yellow No. 5 (Tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the ...
-
ADVERSE REACTIONSDue to oral minocycline’s virtually complete absorption, side effects to the lower bowel, particularly diarrhea, have been infrequent. The following adverse reactions have been observed in ...
-
OVERDOSAGEThe adverse events more commonly seen in overdose are dizziness, nausea, and vomiting. No specific antidote for minocycline is known. In case of overdosage, discontinue medication, treat ...
-
DOSAGE AND ADMINISTRATIONTHE USUAL DOSAGE AND FREQUENCY OF ADMINISTRATION OF MINOCYCLINE DIFFERS FROM THAT OF THE OTHER TETRACYCLINES. EXCEEDING THE RECOMMENDED DOSAGE MAY RESULT IN AN INCREASED INCIDENCE OF SIDE ...
-
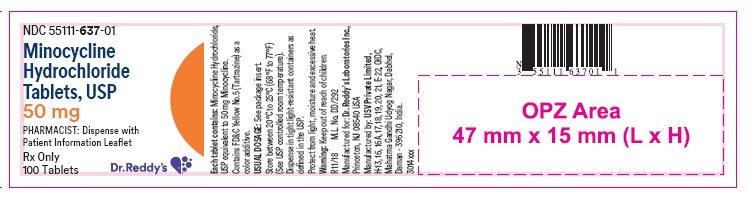
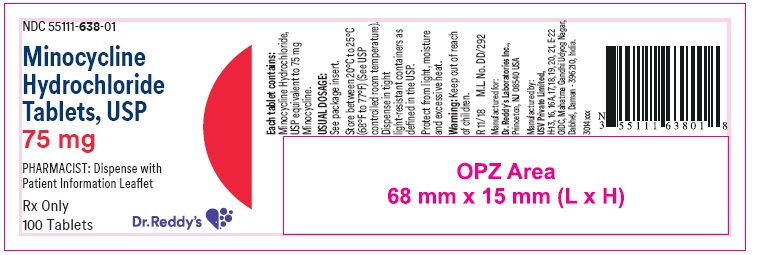
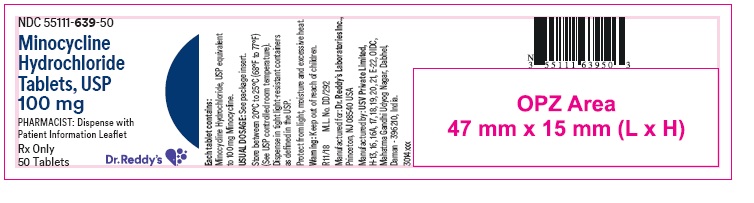
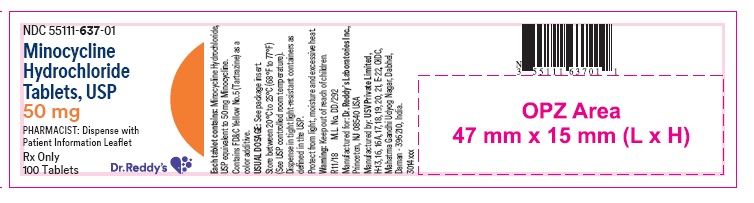
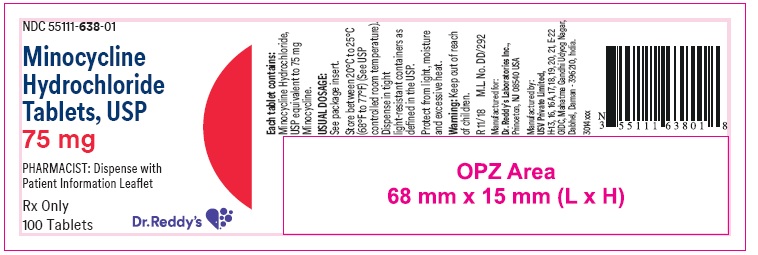
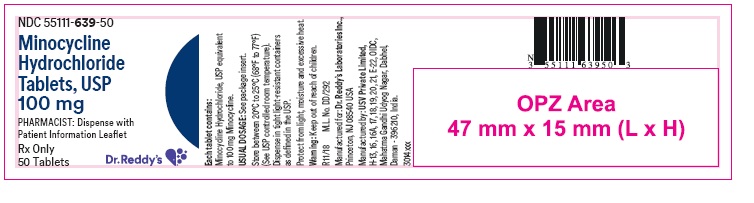
HOW SUPPLIEDMinocycline hydrochloride tablets USP are supplied as aqueous film coated tablets containing minocycline hydrochloride equivalent to 50 mg, 75 mg and 100 mg minocycline. The 50 mg tablets are ...
-
ANIMAL PHARMACOLOGY AND TOXICOLOGYMinocycline hydrochloride has been observed to cause a dark discoloration of the thyroid in experimental animals (rats, minipigs, dogs and monkeys). In the rat, chronic treatment with minocycline ...
-
REFERENCESNational Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically - Fourth Edition; Approved Standard. NCCLS ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Minocycline Hydrochloride Tablets USP, 50 mg, 75 mg, and 100 mg - (mye-no-SYE-kleen) Read the Patient Information that comes with Minocyline Hydrochloride Tablets before you ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL SECTIONContainer label : 50 mg
-
PRINCIPAL DISPLAY PANELContainer label : 75 mg
-
PRINCIPAL DISPLAY PANELContainer label : 100 mg
-
INGREDIENTS AND APPEARANCEProduct Information