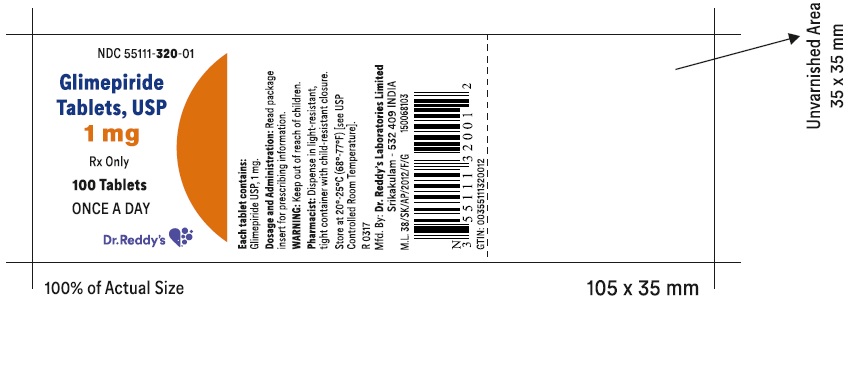
Label: GLIMEPIRIDE tablet
- NDC Code(s): 55111-320-01, 55111-320-05, 55111-320-30, 55111-320-78, view more
- Packager: Dr. Reddy's Laboratories Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 16, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GLIMEPIRIDE TABLETS safely and effectively. See full prescribing information for GLIMEPIRIDE TABLETS. GLIMEPIRIDE tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEGlimepiride tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies (14.1)]. Limitations of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - Glimepiride tablets should be administered with breakfast or the first main meal of the day. The recommended starting dose of glimepiride tablets are 1 mg or 2 mg ...
-
3 DOSAGE FORMS AND STRENGTHSGlimepiride tablets USP, are formulated as tablets of: Glimepiride tablets USP, 1 mg are peach, oval, flat beveled edged, uncoated tablets debossed “RDY” on one side and “320” separating “3” and ...
-
4 CONTRAINDICATIONSGlimepiride tablets are contraindicated in patients with a history of a hypersensitivity reaction to: Glimepiride or any of the product’s ingredients [see Warnings and Precautions (5.2)] ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypoglycemia - All sulfonylureas, including glimepiride, can cause severe hypoglycemia [see Adverse Reactions (6.1)]. The patient's ability to concentrate and react may be impaired as a ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in more detail below and elsewhere in the labeling: Hypoglycemia [see Warnings and Precautions (5.1)] Hemolytic anemia [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Drugs Affecting Glucose Metabolism - A number of medications affect glucose metabolism and may require glimepiride tablets dose adjustment and particularly close monitoring for ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from a small number of published studies and postmarketing experience with glimepiride use in pregnancy over decades have not identified any drug ...
-
10 OVERDOSAGEAn overdosage of glimepiride tablets, as with other sulfonylureas, can produce severe hypoglycemia. Mild episodes of hypoglycemia can be treated with oral glucose. Severe hypoglycemic reactions ...
-
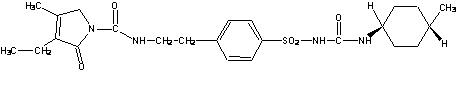
11 DESCRIPTIONGlimepiride tablets USP, are an oral sulfonylurea that contains the active ingredient glimepiride USP. Chemically, glimepiride USP is identified as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Glimepiride primarily lowers blood glucose by stimulating the release of insulin from pancreatic beta cells. Sulfonylureas bind to the sulfonylurea receptor in the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility - Studies in rats at doses of up to 5000 parts per million (ppm) in complete feed (approximately 340 times the maximum recommended human ...
-
14 CLINICAL STUDIES14.1 Monotherapy - A total of 304 patients with type 2 diabetes already treated with sulfonylurea therapy participated in a 14-week, multicenter, randomized, double-blind, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGGlimepiride tablets USP, are available in the following strengths and package sizes: Glimepiride tablets USP, 1 mg are peach, oval, flat beveled edged, uncoated tablets debossed “RDY” on one ...
-
17 PATIENT COUNSELING INFORMATIONHypoglycemia Explain the symptoms and treatment of hypoglycemia as well as conditions that predispose to hypoglycemia. Inform patients that their ability to concentrate and react may be ...
-
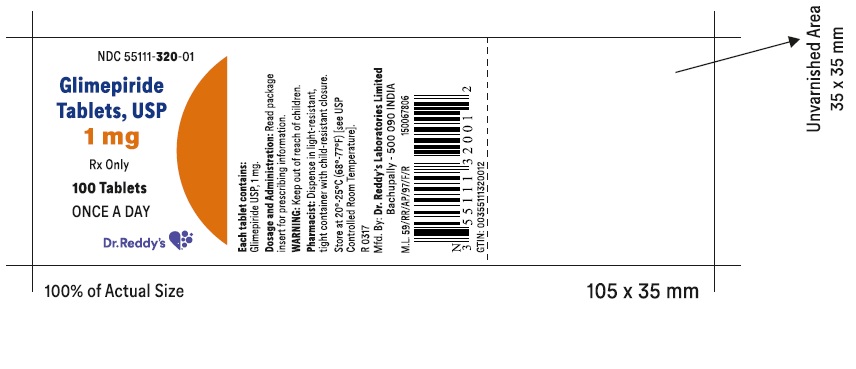
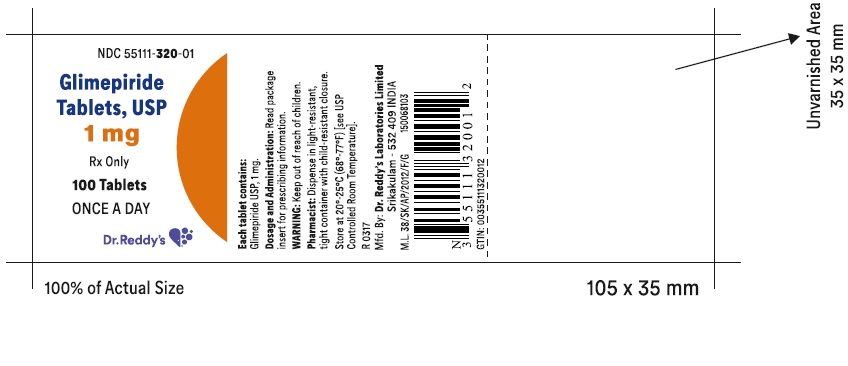
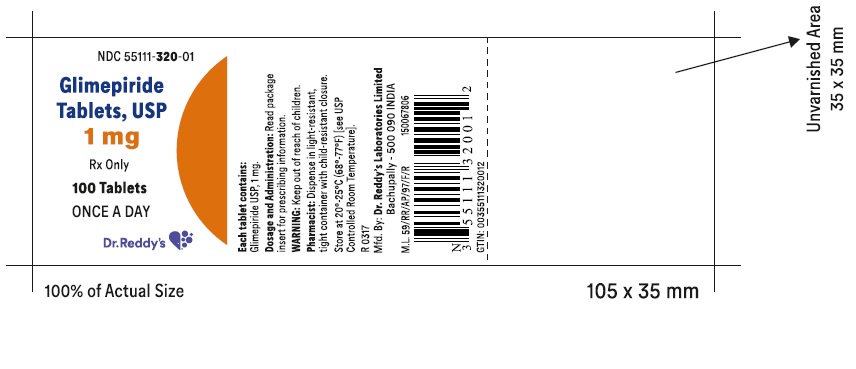
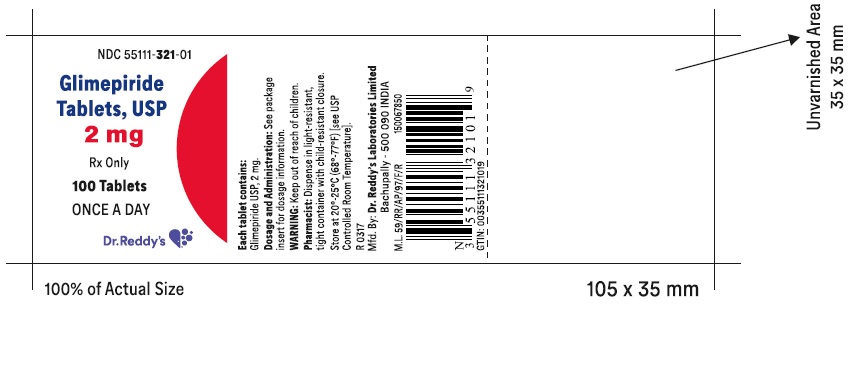
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTIONGlimepiride tablets USP, 1 mg - Container Label - Unvarnished Area consists of: 2D Barcode, Lot Number, Expiry Date and Serial Number.
-
PRINCIPAL DISPLAY PANEL
-
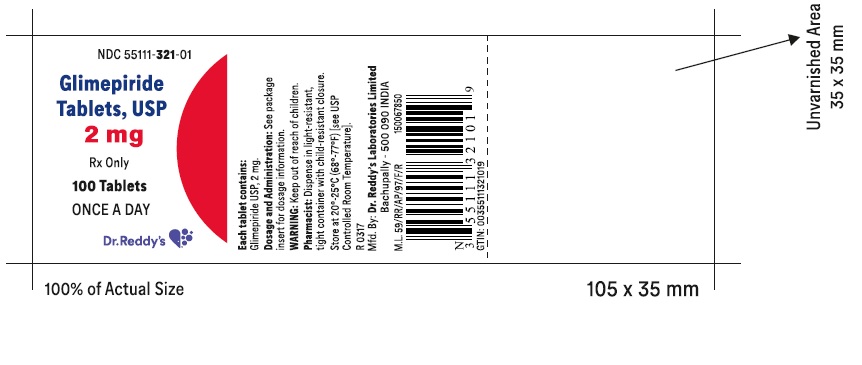
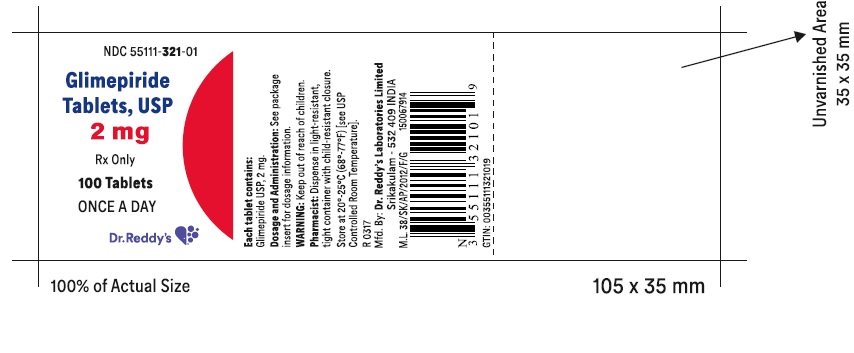
PRINCIPAL DISPLAY PANELGlimepiride tablets USP, 2 mg - Container Label - Unvarnished Area consists of: 2D Barcode, Lot Number, Expiry Date and Serial Number.
-
PRINCIPAL DISPLAY PANEL
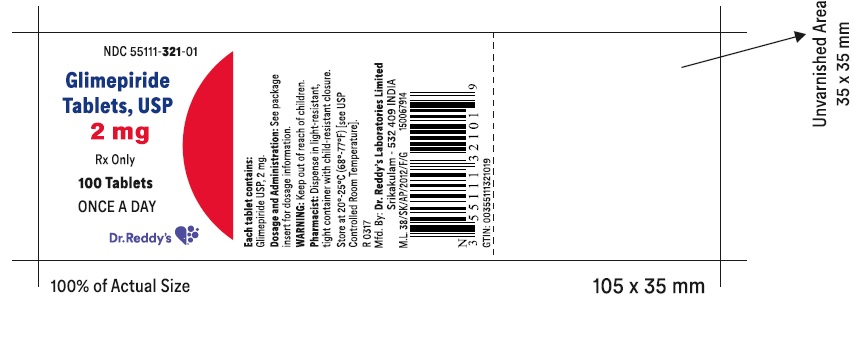
-
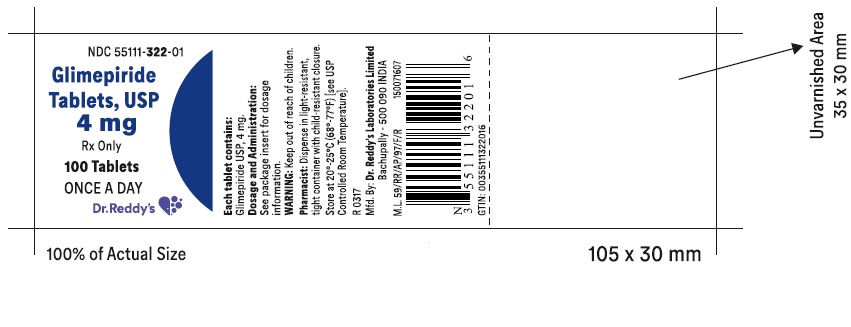
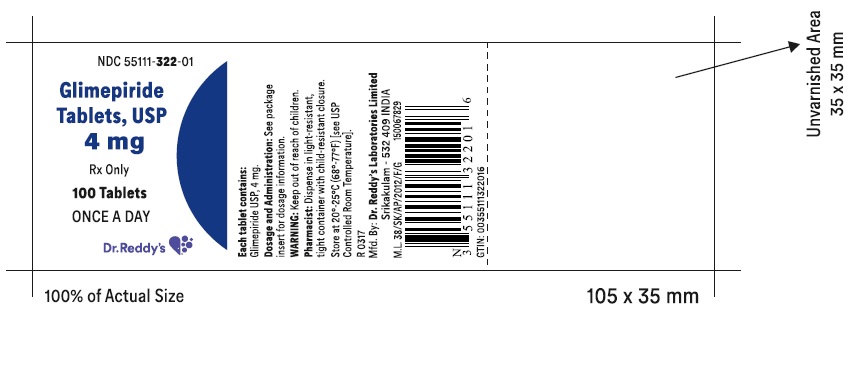
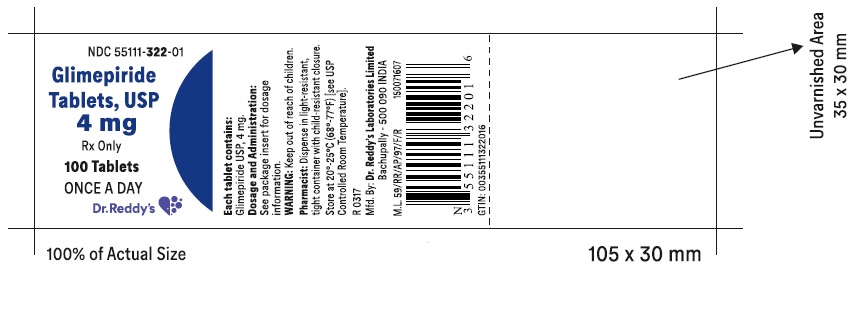
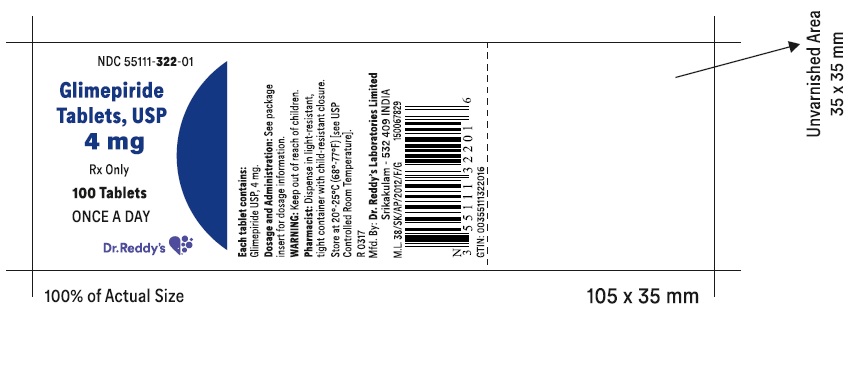
PRINCIPAL DISPLAY PANELGlimepiride tablets USP, 4 mg - Container Label - Unvarnished Area consists of: 2D Barcode, Lot Number, Expiry Date and Serial Number.
-
PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANELGlimepiride Tablets USP, 1 mg - Carton label
-
PRINCIPAL DISPLAY PANELGlimepiride Tablets USP, 2 mg - Carton label
-
PRINCIPAL DISPLAY PANELGlimepiride Tablets USP, 4 mg - Carton label
-
INGREDIENTS AND APPEARANCEProduct Information