Label: SUMATRIPTAN SUCCINATE- sumatriptan succinate tablet
- NDC Code(s): 55111-291-01, 55111-291-05, 55111-291-09, 55111-291-30, view more
- Packager: Dr. Reddy's Laboratories Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 10, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SUMATRIPTAN TABLETS safely and effectively. See full prescribing information for SUMATRIPTAN TABLETS. SUMATRIPTAN tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESumatriptan tablets are indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use: • Use only if a clear diagnosis of migraine headache has been ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended dose of sumatriptan tablets are 25 mg, 50 mg, or 100 mg. Doses of 50 mg and 100 mg may provide a greater effect than the 25-mg dose, but doses of ...
-
3 DOSAGE FORMS AND STRENGTHS25 mg Tablets: White, round, biconvex film-coated tablets debossed with “RDY” on one side and “291” on the other side. 50 m g Tablets: White, round, biconvex film-coated tablets debossed ...
-
4 CONTRAINDICATIONSSumatriptan tablets are contraindicated in patients with: • Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal’s Angina - The use of sumatriptan tablets are contraindicated in patients with ischemic or vasospastic CAD. There have been ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the prescribing information: • Myocardial ischemia, myocardial infarction, and Prinzmetal’s angina [see Warnings ...
-
7 DRUG INTERACTIONS7.1 Ergot-Containing Drugs - Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Data from a prospective pregnancy exposure registry and epidemiological studies of pregnant women have not detected an increased frequency of birth defects or a ...
-
10 OVERDOSAGEPatients in clinical trials (N = 670) received single oral doses of 140 to 300 mg without significant adverse reactions. Volunteers (N = 174) received single oral doses of 140 to 400 mgwithout ...
-
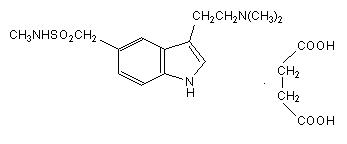
11 DESCRIPTIONSumatriptan tablets USP contain sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sumatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine headache ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility - Carcinogenesis - In carcinogenicity studies in mouse and rat, sumatriptan was administered orally for 78 and 104 weeks, respectively ...
-
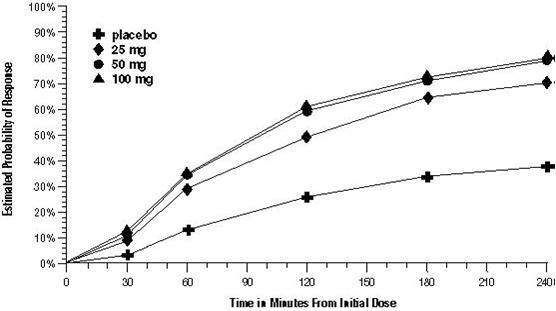
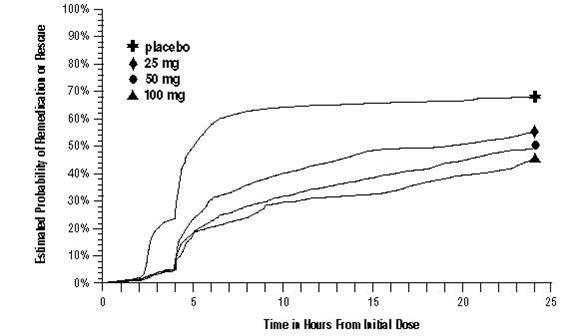
14 CLINICAL STUDIESThe efficacy of sumatriptan tablets in the acute treatment of migraine headaches was demonstrated in 3, randomized, double-blind, placebo-controlled trials. Patients enrolled in these 3 trials ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSumatriptan tablets USP, 25, 50, and 100 mg of sumatriptan (base) as the succinate. Sumatriptan tablets USP, 25 mg are white, round, biconvex film-coated tablets debossed with “RDY” on one side ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Risk of Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other Vasospasm-Related Events ...
-
PATIENT PACKAGE INSERTPATIENTINFORMATION - Sumatriptan Tablets USP - (soo'' ma trip' tan) What is the most important information I should know about sumatriptan tablets? Sumatriptan tablets can cause serious ...
-
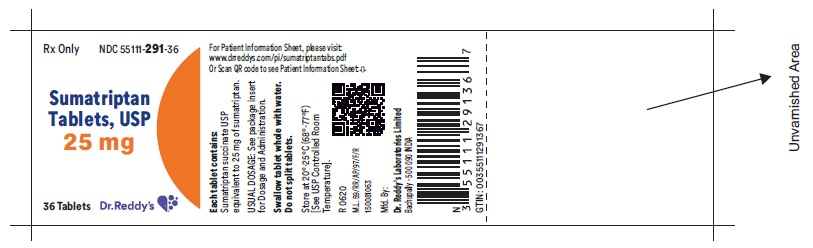
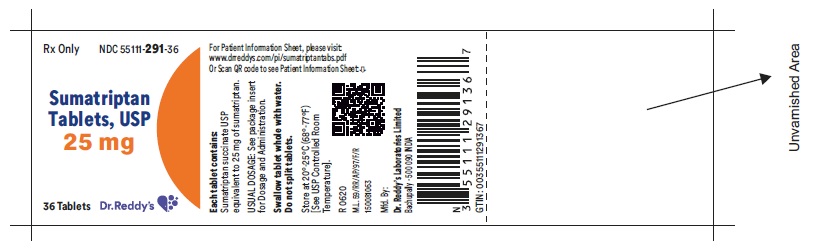
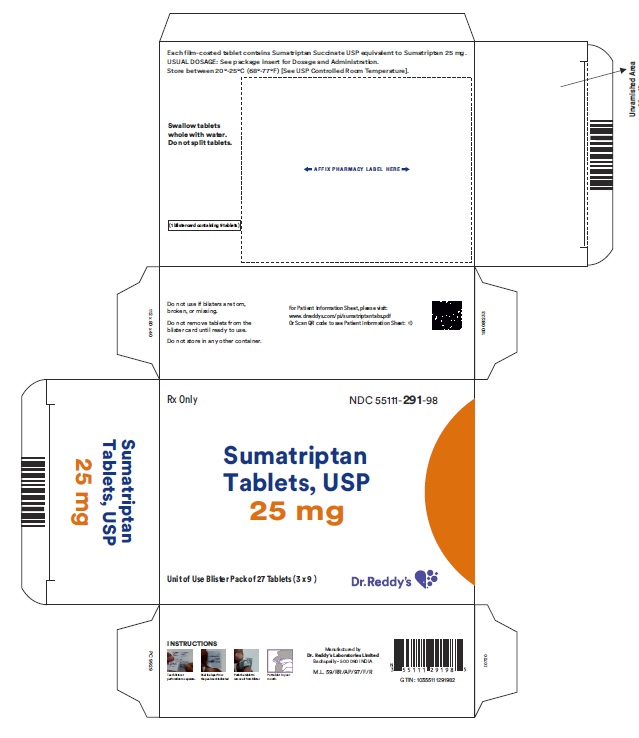
Package Label Principal Display PanelUnvarinshed Area Consists of: 2D Barcode, Lot Number, Expiry Date and Serial Number - 25 mg : Container label
-
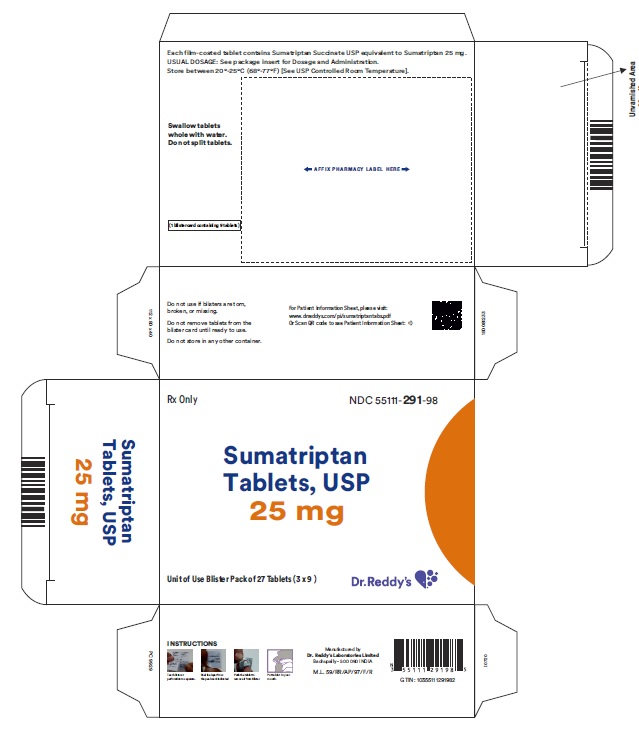
PRINCIPAL DISPLAY PANEL25 mg : Carton label
-
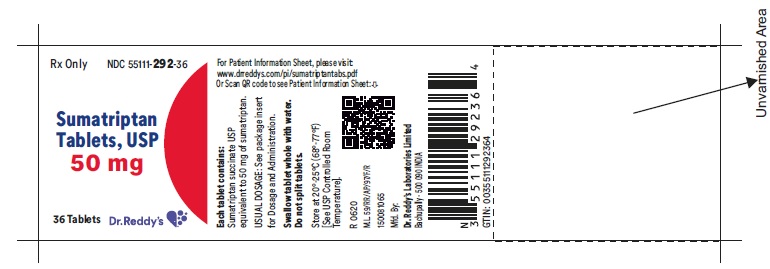
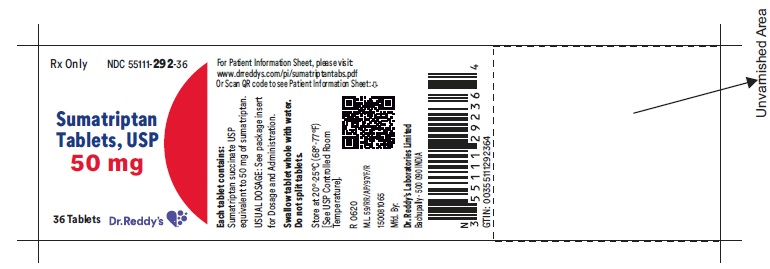
PRINCIPAL DISPLAY PANEL50 mg : Container label
-
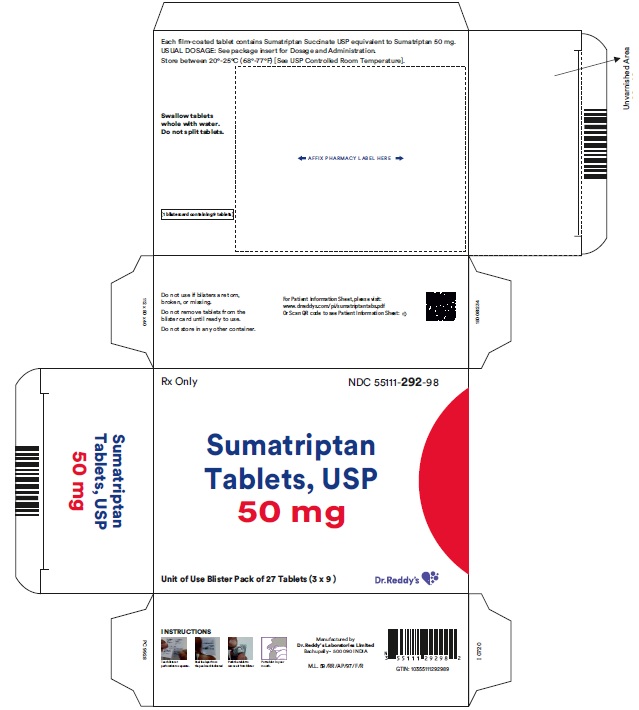
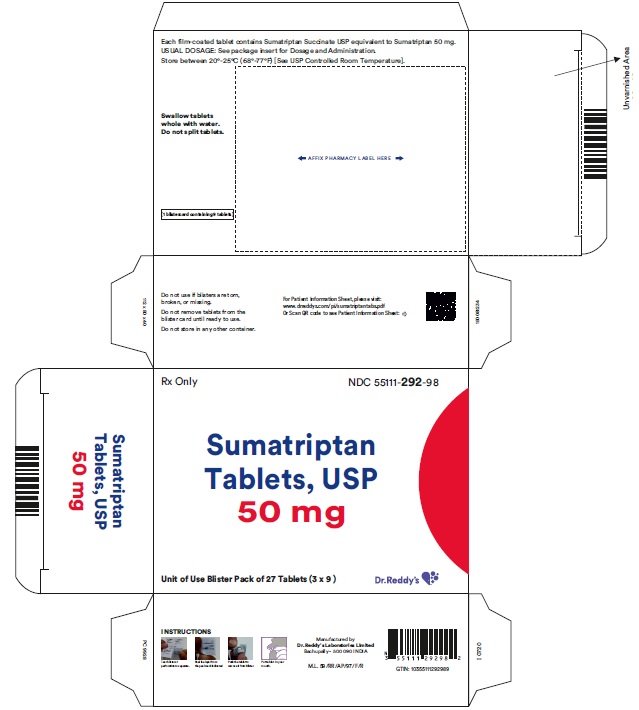
PRINCIPAL DISPLAY PANEL50 mg : Carton label
-
PRINCIPAL DISPLAY PANEL100 mg : Container label
-
PRINCIPAL DISPLAY PANEL100 mg : Carton label
-
INGREDIENTS AND APPEARANCEProduct Information