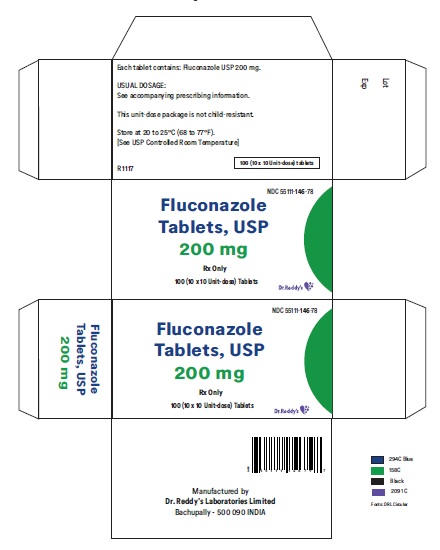
Label: FLUCONAZOLE tablet
-
NDC Code(s):
55111-143-01,
55111-143-05,
55111-143-30,
55111-143-78, view more55111-143-79, 55111-144-01, 55111-144-05, 55111-144-30, 55111-144-78, 55111-144-79, 55111-145-12, 55111-145-71, 55111-146-01, 55111-146-05, 55111-146-30, 55111-146-78, 55111-146-79
- Packager: Dr. Reddy's Laboratories Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONFluconazole, the first of a new subclass of synthetic triazole antifungal agents, is available as tablets for oral administration. Fluconazole is designated chemically as ...
Fluconazole, the first of a new subclass of synthetic triazole antifungal agents, is available as tablets for oral administration.
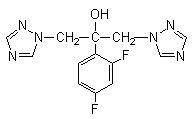
Fluconazole is designated chemically as 2,4-difluoro-α,α1-bis(1H-1,2,4-triazol-1-ylmethyl) benzyl alcohol with a molecular formula of C13H12F2N6O and molecular weight of 306.3. The structural formula is:
Fluconazole, USP is a white or almost white crystalline powder, which is freely soluble in methanol, soluble in alcohol and in acetone, sparingly soluble in isopropyl alcohol and in chloroform. Slightly soluble in water, very slightly soluble in toluene.
Fluconazole tablets, USP contain 50 mg, 100 mg, 150 mg, or 200 mg of fluconazole, USP and the following inactive ingredients: dibasic calcium phosphate anhydrous, ferric oxide (iron oxide, red), magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate, and starch.
Fluconazole tablets meets USP Dissolution Test 2.
Close -
CLINICAL PHARMACOLOGYPharmacokinetics and Metabolism - The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the ...
Pharmacokinetics and Metabolism
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmax of 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmax and AUC data from a food-effect study involving administration of fluconazole to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Table 1: Mean Pharmacokinetic Parameters of Fluconazole in Adult Healthy Volunteers Following the Administration of Fluconazole
Dose regimen Cmax (mcg/mL) AUC0-24 (mcg*h/mL) Half-life (hours) 50 mg oral (once daily x 7 days) 2.21 37.6 26.6 100 mg oral (once daily x 7 days) 4.81 82.5 27.7 150 mg single oral 2.70 137* 34.1 200 mg oral (once daily x 14 days) 10.12 169.5 31 300 mg oral (once daily x 14 days) 15.98 299.4 34 400 mg oral (once daily x 14 days) 18.89 349.9 31 *AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Table 2: Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration
Tissue or Fluid Ratio of Fluconazole
Tissue (Fluid)/Plasma Concentration*Cerebrospinal fluid† 0.5 to 0.9 Saliva 1 Sputum 1 Blister fluid 1 Urine 10 Normal skin 10 Nails 1 Blister skin 2 Vaginal tissue 1 Vaginal fluid 0.4 to 0.7 * Relative to concurrent concentrations in plasma in subjects with normal renal function.
† Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See DOSAGE AND ADMINISTRATION.) A 3-hour hemodialysis session decreases plasma concentrations by approximately 50%.
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
Pharmacokinetics in Pediatric Patients
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Table 3: Pharmacokinetic Parameters* of Fluconazole in Pediatric Patients Following the Administration of Fluconazole
AgeStudied Dose
(mg/kg)Clearance
(mL/min/kg)Half-life
(Hours)Cmax
(mcg/mL)AUC
(mcg*h/mL)Vdss
(L/kg)2 to 60 days IV 25 mg/kg on day one
followed by IV 12 mg/kg once daily0.29 (35%)
N=854.2 23.4 (29%)
N=8439 (25%)^ 1.13 (31%) 9 months to 13 years Single-Oral 2 mg/kg 0.40 (38%)
N=1425.0 2.9 (22%)
N=1694.7 (34%)*
N=14N/A 9 months to 13 years Single-Oral 8 mg/kg 0.51 (60%)
N=1519.5 9.8 (20%)
N=15362.5 (58%)*
N=14N/A 5 to 15 years Multiple IV 2 mg/kg 0.49 (40%)
N=417.4 5.5 (25%)
N=567.4 (26%)^
N=40.722 (36%)
N=45 to 15 years Multiple IV 4 mg/kg 0.59 (64%)
N=515.2 11.4 (44%)
N=6139.1 (46%)^
N=50.729 (33%)
N=55 to 15 years Multiple IV 8 mg/kg 0.66 (31%)
N=717.6 14.1 (22%)
N=8196.7 (25%)^
N=71.069 (37%)
N=7*Data for Clearance, Cmax, AUC and Vdss are presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.180 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24 of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24 >400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.)
Pharmacokinetics in Elderly
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmax was 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg∙h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject's terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life – creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
Drug Interaction Studies
(See PRECAUTIONS, Drug Interactions)
Oral contraceptives: Oral contraceptives were administered as a single dose both before and after the oral administration of fluconazole 50 mg once daily for 10 days in 10 healthy women. There was no significant difference in ethinyl estradiol or levonorgestrel AUC after the administration of 50 mg of fluconazole. The mean increase in ethinyl estradiol AUC was 6% (range: -47 to 108%) and levonorgestrel AUC increased 17% (range: -33 to 141%).
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: -12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: -11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Cimetidine: Fluconazole 100 mg was administered as a single oral dose alone and two hours after a single dose of cimetidine 400 mg to six healthy male volunteers. After the administration of cimetidine, there was a significant decrease in fluconazole AUC and Cmax. There was a mean ± SD decrease in fluconazole AUC of 13% ± 11% (range: -3.4 to -31%) and Cmax decreased 19% ± 14% (range: -5 to -40%). However, the administration of cimetidine 600 mg to 900 mg intravenously over a four-hour period (from one hour before to 3 hours after a single oral dose of fluconazole 200 mg) did not affect the bioavailability or pharmacokinetics of fluconazole in 24 healthy male volunteers.
Antacid: Administration of Maalox® (20 mL) to 14 normal male volunteers immediately prior to a single dose of fluconazole 100 mg had no effect on the absorption or elimination of fluconazole.
Hydrochlorothiazide: Concomitant oral administration of 100 mg fluconazole and 50 mg hydrochlorothiazide for 10 days in 13 normal volunteers resulted in a significant increase in fluconazole AUC and Cmax compared to fluconazole given alone. There was a mean ± SD increase in fluconazole AUC and Cmax of 45% ± 31% (range: 19 to 114%) and 43% ± 31% (range: 19 to 122%), respectively. These changes are attributed to a mean ± SD reduction in renal clearance of 30% ± 12% (range: -10 to -50%).
Rifampin: Administration of a single oral 200 mg dose of fluconazole after 15 days of rifampin administered as 600 mg daily in eight healthy male volunteers resulted in a significant decrease in fluconazole AUC and a significant increase in apparent oral clearance of fluconazole. There was a mean ± SD reduction in fluconazole AUC of 23% ± 9% (range: -13 to -42%). Apparent oral clearance of fluconazole increased 32% ± 17% (range: 16 to 72%). Fluconazole half-life decreased from 33.4 ± 4.4 hours to 26.8 ± 3.9 hours. (See PRECAUTIONS.)
Warfarin: There was a significant increase in prothrombin time response (area under the prothrombin time-time curve) following a single dose of warfarin (15 mg) administered to 13 normal male volunteers following oral fluconazole 200 mg administered daily for 14 days as compared to the administration of warfarin alone. There was a mean ± SD increase in the prothrombin time response (area under the prothrombin time-time curve) of 7% ± 4% (range: -2 to 13%). (See PRECAUTIONS.) Mean is based on data from 12 subjects as one of 13 subjects experienced a 2-fold increase in his prothrombin time response.
Phenytoin: Phenytoin AUC was determined after 4 days of phenytoin dosing (200 mg daily, orally for 3 days followed by 250 mg intravenously for one dose) both with and without the administration of fluconazole (oral fluconazole 200 mg daily for 16 days) in 10 normal male volunteers. There was a significant increase in phenytoin AUC. The mean ± SD increase in phenytoin AUC was 88% ± 68% (range: 16 to 247%). The absolute magnitude of this interaction is unknown because of the intrinsically nonlinear disposition of phenytoin. (See PRECAUTIONS.)
Cyclosporine: Cyclosporine AUC and Cmax were determined before and after the administration of fluconazole 200 mg daily for 14 days in eight renal transplant patients who had been on cyclosporine therapy for at least 6 months and on a stable cyclosporine dose for at least 6 weeks. There was a significant increase in cyclosporine AUC, Cmax, Cmin (24-hour concentration), and a significant reduction in apparent oral clearance following the administration of fluconazole. The mean ± SD increase in AUC was 92% ± 43% (range: 18 to 147%). The Cmax increased 60% ± 48% (range: -5 to 133%). The Cmin increased 157% ± 96% (range: 33 to 360%). The apparent oral clearance decreased 45% ± 15% (range: -15 to -60%). (See PRECAUTIONS.)
Zidovudine: Plasma zidovudine concentrations were determined on two occasions (before and following fluconazole 200 mg daily for 15 days) in 13 volunteers with AIDS or ARC who were on a stable zidovudine dose for at least two weeks. There was a significant increase in zidovudine AUC following the administration of fluconazole. The mean ± SD increase in AUC was 20% ± 32% (range: -27 to 104%). The metabolite, GZDV, to parent drug ratio significantly decreased after the administration of fluconazole, from 7.6 ± 3.6 to 5.7 ± 2.2.
Theophylline: The pharmacokinetics of theophylline were determined from a single intravenous dose of aminophylline (6 mg/kg) before and after the oral administration of fluconazole 200 mg daily for 14 days in 16 normal male volunteers. There were significant increases in theophylline AUC, Cmax, and half-life with a corresponding decrease in clearance. The mean ± SD theophylline AUC increased 21% ± 16% (range: -5 to 48%). The Cmax increased 13% ± 17% (range: -13 to 40%). Theophylline clearance decreased 16% ± 11% (range: -32 to 5%). The half-life of theophylline increased from 6.6 ± 1.7 hours to 7.9 ± 1.5 hours. (See PRECAUTIONS.)
Quinidine: Although not studied in vitro or in vivo, concomitant administration of fluconazole with quinidine may result in inhibition of quinidine metabolism. Use of quinidine has been associated with QT prolongation and rare occurrences of torsade de pointes.Coadministration of fluconazole and quinidine is contraindicated.(See CONTRAINDICATIONSand PRECAUTIONS.)
Oral hypoglycemics: The effects of fluconazole on the pharmacokinetics of the sulfonylurea oral hypoglycemic agents tolbutamide, glipizide, and glyburide were evaluated in three placebo-controlled studies in normal volunteers. All subjects received the sulfonylurea alone as a single dose and again as a single dose following the administration of fluconazole 100 mg daily for 7 days. In these three studies, 22/46 (47.8%) of fluconazole-treated patients and 9/22 (40.1%) of placebo-treated patients experienced symptoms consistent with hypoglycemia. (See PRECAUTIONS.)
Tolbutamide: In 13 normal male volunteers, there was significant increase in tolbutamide (500 mg single dose) AUC and Cmax following the administration of fluconazole. There was a mean ± SD increase in tolbutamide AUC of 26% ± 9% (range: 12 to 39%). Tolbutamide Cmax increased 11% ± 9% (range: -6 to 27%). (See PRECAUTIONS.)
Glipizide: The AUC and Cmax of glipizide (2.5 mg single dose) were significantly increased following the administration of fluconazole in 13 normal male volunteers. There was a mean ± SD increase in AUC of 49% ± 13% (range: 27 to 73%) and an increase in Cmax of 19% ± 23% (range: -11 to 79%). (See PRECAUTIONS.)
Glyburide: The AUC and Cmax of glyburide (5 mg single dose) were significantly increased following the administration of fluconazole in 20 normal male volunteers. There was a mean ± SD increase in AUC of 44% ± 29% (range: -13 to 115%) and Cmax increased 19% ± 19% (range: -23 to 62%). Five subjects required oral glucose following the ingestion of glyburide after 7 days of fluconazole administration. (See PRECAUTIONS.)
Rifabutin: There have been published reports that an interaction exists when fluconazole is administered concomitantly with rifabutin, leading to increased serum levels of rifabutin. (See PRECAUTIONS.)
Tacrolimus: There have been published reports that an interaction exists when fluconazole is administered concomitantly with tacrolimus, leading to increased serum levels of tacrolimus. (See PRECAUTIONS.)
Midazolam: The effect of fluconazole on the pharmacokinetics and pharmacodynamics of midazolam was examined in a randomized, cross-over study in 12 volunteers. In the study, subjects ingested placebo or 400 mg fluconazole on Day 1 followed by 200 mg daily from Day 2 to Day 6. In addition, a 7.5 mg dose of midazolam was orally ingested on the first day, 0.05 mg/kg was administered intravenously on the fourth day, and 7.5 mg orally on the sixth day. Fluconazole reduced the clearance of IV midazolam by 51%. On the first day of dosing, fluconazole increased the midazolam AUC and Cmax by 259% and 150%, respectively. On the sixth day of dosing, fluconazole increased the midazolam AUC and Cmax by 259% and 74%, respectively. The psychomotor effects of midazolam were significantly increased after oral administration of midazolam but not significantly affected following intravenous midazolam.
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmax of midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmax by 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmax by 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See PRECAUTIONS.)
Azithromycin: An open-label, randomized, three-way crossover study in 18 healthy subjects assessed the effect of a single 800 mg oral dose of fluconazole on the pharmacokinetics of a single 1,200 mg oral dose of azithromycin as well as the effects of azithromycin on the pharmacokinetics of fluconazole. There was no significant pharmacokinetic interaction between fluconazole and azithromycin.
Voriconazole: Voriconazole is a substrate for both CYP2C9 and CYP3A4 isoenzymes. Concurrent administration of oral Voriconazole (400 mg Q12h for 1 day, then 200 mg Q12h for 2.5 days) and oral fluconazole (400 mg on Day 1, then 200 mg Q24h for 4 days) to 6 healthy male subjects resulted in an increase in Cmax and AUCτ of voriconazole by an average of 57% (90% CI: 20% to 107%) and 79% (90% CI: 40% to 128%), respectively. In a follow-on clinical study involving 8 healthy male subjects, reduced dosing and/or frequency of voriconazole and fluconazole did not eliminate or diminish this effect. (See PRECAUTIONS.)
Tofacitinib: Coadministration of fluconazole (400 mg on Day 1 and 200 mg once daily for 6 days [Days 2 to 7]) and tofacitinib (30 mg single dose on Day 5) in healthy subjects resulted in increased mean tofacitinib AUC and Cmax values of approximately 79% (90% CI: 64% to 96%) and 27% (90% CI: 12% to 44%), respectively, compared to administration of tofacitinib alone. (See PRECAUTIONS.)
Abrocitinib: When co-administered with fluconazole (inhibitor of CYP2C9, 2C19, and 3A4), the systemic exposure (AUC) of abrocitinib was approximately 4.8-fold higher and the combined exposure (AUC) of abrocitinib and its active metabolites was approximately 2.5-fold higher compared to when abrocitinib was administered alone (See PRECAUTIONS).
CloseMicrobiology
Mechanism of Action
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (ERG11) encoding for the target enzyme lead to an altered target with decreased affinity for azoles. Overexpression of ERG11 results in the production of high concentrations of the target enzyme, creating the need for higher intracellular drug concentrations to inhibit all of the enzyme molecules in the cell.
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by MDR genes) and those of the ATP-binding cassette superfamily (encoded by CDR genes). Upregulation of the MDR gene leads to fluconazole resistance, whereas, upregulation of CDR genes may lead to resistance to multiple azoles.
Resistance in Candida glabrata usually includes upregulation of CDR genes resulting in resistance to multiple azoles. For an isolate where the minimum inhibitory concentration (MIC) is categorized as Intermediate (16 to 32 mcg/mL), the highest fluconazole dose is recommended.
Antimicrobial Activity
Fluconazole has been shown to be active against most isolates of the following microorganisms both in vitro and in clinical infections.
Candida albicans
Candida glabrata (Many isolates are intermediately susceptible)
Candida parapsilosis
Candida tropicalis
Cryptococcus neoformans
The following in vitro data are available, but their clinical significance is unknown. At least 90% of the following fungi exhibit an in vitro MIC less than or equal to the susceptible breakpoint for fluconazole (https://www.fda.gov/STIC) against isolates of similar genus or organism group. However, the effectiveness of fluconazole in treating clinical infections due to these fungi has not been established in adequate and well-controlled clinical trials.
Candida dubliniensis
Candida guilliermondii
Candida kefyr
Candida lusitaniae
Candida krusei should be considered to be resistant to fluconazole. Resistance in C. krusei appears to be mediated by reduced sensitivity of the target enzyme to inhibition by the agent.
-
INDICATIONS AND USAGEFluconazole tablets are indicated for the treatment of: 1. Vaginal candidiasis (vaginal yeast infections due to Candida) 2. Oropharyngeal and esophageal candidiasis. In open noncomparative ...
Fluconazole tablets are indicated for the treatment of:
1. Vaginal candidiasis (vaginal yeast infections due to Candida)
2. Oropharyngeal and esophageal candidiasis. In open noncomparative studies of relatively small numbers of patients, fluconazole tablets were also effective for the treatment of Candida urinary tract infections, peritonitis, and systemic Candida infections including candidemia, disseminated candidiasis, and pneumonia.
3. Cryptococcal meningitis. Before prescribing fluconazole tablets for AIDS patients with cryptococcal meningitis, please see CLINICAL STUDIES section. Studies comparing fluconazole tablets to amphotericin B in non-HIV infected patients have not been conducted.
Prophylaxis: Fluconazole tablets are also indicated to decrease the incidence of candidiasis in patients undergoing bone marrow transplantation who receive cytotoxic chemotherapy and/or radiation therapy.
Specimens for fungal culture and other relevant laboratory studies (serology, histopathology) should be obtained prior to therapy to isolate and identify causative organisms. Therapy may be instituted before the results of the cultures and other laboratory studies are known; however, once these results become available, anti-infective therapy should be adjusted accordingly.
Close -
CLINICAL STUDIESCryptococcal meningitis - In a multicenter study comparing fluconazole (200 mg/day) to amphotericin B (0.3 mg/kg/day) for treatment of cryptococcal meningitis in patients with AIDS, a ...
Cryptococcal meningitis
In a multicenter study comparing fluconazole (200 mg/day) to amphotericin B (0.3 mg/kg/day) for treatment of cryptococcal meningitis in patients with AIDS, a multivariate analysis revealed three pretreatment factors that predicted death during the course of therapy: abnormal mental status, cerebrospinal fluid cryptococcal antigen titer greater than 1:1024, and cerebrospinal fluid white blood cell count of less than 20 cells/mm3. Mortality among high risk patients was 33% and 40% for amphotericin B and fluconazole patients, respectively (p=0.58), with overall deaths 14% (9 of 63 subjects) and 18% (24 of 131 subjects) for the 2 arms of the study (p=0.48). Optimal doses and regimens for patients with acute cryptococcal meningitis and at high risk for treatment failure remain to be determined. (Saag, et al. N Engl J Med 1992; 326:83-9.)
Vaginal candidiasis
Two adequate and well-controlled studies were conducted in the U.S. using the 150 mg tablet. In both, the results of the fluconazole regimen were comparable to the control regimen (clotrimazole or miconazole intravaginally for 7 days) both clinically and statistically at the one month post-treatment evaluation.
The therapeutic cure rate, defined as a complete resolution of signs and symptoms of vaginal candidiasis (clinical cure), along with a negative KOH examination and negative culture for Candida (microbiologic eradication), was 55% in both the fluconazole group and the vaginal products group.
Fluconazole PO 150 mg tablet Vaginal Product qhs × 7 days Enrolled 448 422 Evaluable at Late Follow-up 347 (77%) 327 (77%) Clinical cure 239/347 (69%) 235/327 (72%) Mycologic eradication 213/347 (61%) 196/327 (60%) Therapeutic cure 190/347 (55%) 179/327 (55%) Approximately three-fourths of the enrolled patients had acute vaginitis (<4 episodes/12 months) and achieved 80% clinical cure, 67% mycologic eradication, and 59% therapeutic cure when treated with a 150 mg fluconazole tablet administered orally. These rates were comparable to control products. The remaining one-fourth of enrolled patients had recurrent vaginitis (≥4 episodes/12 months) and achieved 57% clinical cure, 47% mycologic eradication, and 40% therapeutic cure. The numbers are too small to make meaningful clinical or statistical comparisons with vaginal products in the treatment of patients with recurrent vaginitis.
Substantially more gastrointestinal events were reported in the fluconazole group compared to the vaginal product group. Most of the events were mild to moderate. Because fluconazole was given as a single dose, no discontinuations occurred.
Parameter Fluconazole PO Vaginal Products Evaluable patients 448 422 With any adverse event 141 (31%) 112 (27%) Nervous System 90 (20%) 69 (16%) Gastrointestinal 73 (16%) 18 (4%) With drug-related event 117 (26%) 67 (16%) Nervous System 61 (14%) 29 (7%) Headache 58 (13%) 28 (7%) Gastrointestinal 68 (15%) 13 (3%) Abdominal pain 25 (6%) 7 (2%) Nausea 30 (7%) 3 (1%) Diarrhea 12 (3%) 2 (<1%) Application site event 0 (0%) 19 (5%) Taste Perversion 6 (1%) 0 (0%) ClosePediatric Studies
Oropharyngeal candidiasis: An open-label, comparative study of the efficacy and safety of fluconazole (2 to 3 mg/kg/day) and oral nystatin (400,000 I.U. 4 times daily) was conducted in immunocompromised pediatric patients from 6 months to 13 years of age with oropharyngeal candidiasis. Clinical and mycological response rates were higher in pediatric patients treated with fluconazole.
Clinical cure at the end of treatment was reported for 86% of fluconazole-treated patients compared to 46% of nystatin treated patients. Mycologically, 76% of fluconazole treated patients had the infecting organism eradicated compared to 11% for nystatin treated patients.
Fluconazole Nystatin Enrolled 96 90 Clinical Cure 76/88 (86%) 36/78 (46%) Mycological eradication** 55/72 (76%) 6/54 (11%) * Subjects without follow-up cultures for any reason were considered nonevaluable for mycological response.
The proportion of patients with clinical relapse 2 weeks after the end of treatment was 14% for subjects receiving fluconazole and 16% for subjects receiving nystatin. At 4 weeks after the end of treatment, the percentages of patients with clinical relapse were 22% for fluconazole and 23% for nystatin.
-
CONTRAINDICATIONSFluconazole tablets are contraindicated in patients who have shown hypersensitivity to fluconazole or to any of its excipients. There is no information regarding cross-hypersensitivity between ...
Fluconazole tablets are contraindicated in patients who have shown hypersensitivity to fluconazole or to any of its excipients. There is no information regarding cross-hypersensitivity between fluconazole and other azole antifungal agents. Caution should be used in prescribing fluconazole to patients with hypersensitivity to other azoles. Coadministration of other drugs known to prolong the QT interval and which are metabolized via the enzyme CYP3A4 such as erythromycin, pimozide, and quinidine are contraindicated in patients receiving fluconazole. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies and PRECAUTIONS.)
Close -
WARNINGS(1) Hepatic injury: Fluconazole tablets should be administered with caution to patients with liver dysfunction. Fluconazole has been associated with rare cases of serious hepatic toxicity ...
(1) Hepatic injury: Fluconazole tablets should be administered with caution to patients with liver dysfunction. Fluconazole has been associated with rare cases of serious hepatic toxicity, including fatalities primarily in patients with serious underlying medical conditions. In cases of fluconazole-associated hepatotoxicity, no obvious relationship to total daily dose, duration of therapy, sex, or age of the patient has been observed. Fluconazole hepatotoxicity has usually, but not always, been reversible on discontinuation of therapy. Patients who develop abnormal liver function tests during fluconazole therapy should be monitored for the development of more severe hepatic injury. Fluconazole tablets should be discontinued if clinical signs and symptoms consistent with liver disease develop that may be attributable to fluconazole.
(2) Anaphylaxis: In rare cases, anaphylaxis has been reported.
(3) Dermatologic: Exfoliative skin disorders during treatment with fluconazole have been reported. Fatal outcomes have been reported in patients with serious underlying diseases. Patients with deep seated fungal infections who develop rashes during treatment with fluconazole tablets should be monitored closely and the drug discontinued if lesions progress. Fluconazole tablets should be discontinued in patients treated for superficial fungal infection who develop a rash that may be attributed to fluconazole.
(4) Potential for fetal harm There are no adequate and well-controlled clinical trials of fluconazole in pregnant women. Case reports describe a pattern of distinct congenital anomalies in infants exposed in utero to high dose maternal fluconazole (400 to 800 mg/day) during most or all of the first trimester. These reported anomalies are similar to those seen in animal studies. If fluconazole is used during pregnancy or if the patient becomes pregnant while taking the drug, the patient should be informed of the potential hazard to the fetus. Effective contraceptive measures should be considered in women of child-bearing potential who are being treated with fluconazole 400 to 800 mg/day and should continue throughout the treatment period and for approximately 1 week (5 to 6 half-lives) after the final dose. Epidemiological studies suggest a potential risk of spontaneous abortion and congenital abnormalities in infants whose mothers were treated with 150 mg of fluconazole as a single or repeated dose in the first trimester, but these epidemiological studies have limitations and these findings have not been confirmed in controlled clinical trials (See PRECAUTIONS: Pregnancy.)
Close -
PRECAUTIONSGeneral - Some azoles, including fluconazole, have been associated with prolongation of the QT interval on the electrocardiogram. Fluconazole causes QT prolongation via the inhibition of ...
General
Some azoles, including fluconazole, have been associated with prolongation of the QT interval on the electrocardiogram. Fluconazole causes QT prolongation via the inhibition of Rectifier Potassium Channel current (Ikr). The QT prolongation caused by other medicinal products (such as amiodarone) may be amplified via the inhibition of cytochrome P450 (CYP) 3A4 (See PRECAUTIONS: Drug Interactions). During post-marketing surveillance, there have been rare cases of QT prolongation and torsade de pointes in patients taking fluconazole. Most of these reports involved seriously ill patients with multiple confounding risk factors, such as structural heart disease, electrolyte abnormalities, and concomitant medications that may have been contributory. Patients with hypokalemia and advanced cardiac failure are at an increased risk for the occurrence of life-threatening ventricular arrhythmias and torsade de pointes.
Fluconazole tablets should be administered with caution to patients with these potentially proarrhythmic conditions.
Concomitant use of fluconazole and erythromycin has the potential to increase the risk of cardiotoxicity (prolonged QT interval, torsade de pointes) and consequently sudden heart death. This combination should be avoided.
Fluconazole tablets should be administered with caution to patients with renal dysfunction.
Adrenal insufficiency has been reported in patients receiving azoles, including fluconazole. Reversible cases of adrenal insufficiency have been reported in patients receiving fluconazole.
When driving vehicles or operating machines, it should be taken into account that occasionally dizziness or seizures may occur.
There have been reports of cases of superinfection with Candida species other than C. albicans, which are often inherently not susceptible to fluconazole (e.g., Candida krusei). Such cases may require alternative antifungal therapy (see CLINICAL PHARMACOLOGY, Microbiology).
Single Dose
The convenience and efficacy of the single dose oral tablet of fluconazole regimen for the treatment of vaginal yeast infections should be weighed against the acceptability of a higher incidence of drug related adverse events with fluconazole (26%) versus intravaginal agents (16%) in U.S. comparative clinical studies. (See ADVERSE REACTIONS and CLINICAL STUDIES.)
Drug Interactions
(See CONTRAINDICATIONS.) Fluconazole is a moderate CYP2C9 and CYP3A4 inhibitor. Fluconazole is also a strong inhibitor of CYP2C19. Patients treated with fluconazole, who are also concomitantly treated with drugs with a narrow therapeutic window metabolized through CYP2C9 and CYP3A4, should be monitored for adverse reactions associated with the concomitantly administered drugs. In addition to the observed/documented interactions mentioned below, there is a risk of increased plasma concentration of other compounds metabolized by CYP2C9, CYP2C19 and CYP3A4 coadministered with fluconazole. Therefore, caution should be exercised when using these combinations and the patients should be carefully monitored. The enzyme inhibiting effect of fluconazole persists 4 to 5 days after discontinuation of fluconazole treatment due to the long half-life of fluconazole. Clinically or potentially significant drug interactions between fluconazole and the following agents/classes have been observed and are described in greater detail below:
Abrocitinib: Drug interaction studies indicate that when co-administered with fluconazole (strong inhibitor of CYP2C19; moderate inhibitor of CYP2C9 and CYP3A4), the systemic exposure of abrocitinib and its active metabolites increased (See CLINICAL PHARMACOLOGY). Avoid concomitant use of abrocitinib with fluconazole. Refer to the abrocitinib Prescribing Information for additional details.
Alfentanil: A study observed a reduction in clearance and distribution volume as well as prolongation of t½ of alfentanil following concomitant treatment with fluconazole. A possible mechanism of action is fluconazole’s inhibition of CYP3A4. Dosage adjustment of alfentanil may be necessary.
Amiodarone: Concomitant administration of fluconazole with amiodarone may increase QT prolongation. Caution must be exercised if the concomitant use of fluconazole and amiodarone is necessary, notably with high-dose fluconazole (800 mg).
Amitriptyline, nortriptyline: Fluconazole increases the effect of amitriptyline and nortriptyline. 5-Nortriptyline and/or S-amitriptyline may be measured at initiation of the combination therapy and after 1 week. Dosage of amitriptyline/nortriptyline should be adjusted, if necessary.
Amphotericin B: Concurrent administration of fluconazole and amphotericin B in infected normal and immunosuppressed mice showed the following results: a small additive antifungal effect in systemic infection with Candida albicans, no interaction in intracranial infection with Cryptococcus neoformans, and antagonism of the two drugs in systemic infection with A. fumigatus. The clinical significance of results obtained in these studies is unknown.
Azithromycin: An open-label, randomized, three-way crossover study in 18 healthy subjects assessed the effect of a single 1,200 mg oral dose of azithromycin on the pharmacokinetics of a single 800 mg oral dose of fluconazole as well as the effects of fluconazole on the pharmacokinetics of azithromycin. There was no significant pharmacokinetic interaction between fluconazole and azithromycin.
Calcium channel blockers: Certain calcium channel antagonists (nifedipine, isradipine, amlodipine, verapamil, and felodipine) are metabolized by CYP3A4. Fluconazole has the potential to increase the systemic exposure of the calcium channel antagonists. Frequent monitoring for adverse events is recommended.
Carbamazepine: Fluconazole inhibits the metabolism of carbamazepine and an increase in serum carbamazepine of 30% has been observed. There is a risk of developing carbamazepine toxicity. Dosage adjustment of carbamazepine may be necessary depending on concentration measurements/effect.
Celecoxib: During concomitant treatment with fluconazole (200 mg daily) and celecoxib (200 mg), the celecoxib Cmax and AUC increased by 68% and 134%, respectively. Half of the celecoxib dose may be necessary when combined with fluconazole.
Coumarin-type anticoagulants: Prothrombin time may be increased in patients receiving concomitant fluconazole and coumarin-type anticoagulants. In post-marketing experience, as with other azole antifungals, bleeding events (bruising, epistaxis, gastrointestinal bleeding, hematuria, and melena) have been reported in association with increases in prothrombin time in patients receiving fluconazole concurrently with warfarin. Careful monitoring of prothrombin time in patients receiving fluconazole and coumarin-type anticoagulants is recommended. Dose adjustment of warfarin may be necessary. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Cyclophosphamide: Combination therapy with cyclophosphamide and fluconazole results in an increase in serum bilirubin and serum creatinine. The combination may be used while taking increased consideration to the risk of increased serum bilirubin and serum creatinine.
Cyclosporine: Fluconazole significantly increases cyclosporine levels in renal transplant patients with or without renal impairment. Careful monitoring of cyclosporine concentrations and serum creatinine is recommended in patients receiving fluconazole and cyclosporine. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.) This combination may be used by reducing the dosage of cyclosporine depending on cyclosporine concentration.
Fentanyl: One fatal case of possible fentanyl-fluconazole interaction was reported. The author judged that the patient died from fentanyl intoxication. Furthermore, in a randomized crossover study with 12 healthy volunteers, it was shown that fluconazole delayed the elimination of fentanyl significantly. Elevated fentanyl concentration may lead to respiratory depression.
HMG-CoA reductase inhibitors: The risk of myopathy and rhabdomyolysis increases when fluconazole is coadministered with HMG-CoA reductase inhibitors metabolized through CYP3A4, such as atorvastatin and simvastatin, or through CYP2C9, such as fluvastatin (decreased hepatic metabolism of the statin). If concomitant therapy is necessary, the patient should be observed for symptoms of myopathy and rhabdomyolysis and creatinine kinase should be monitored. HMG-CoA reductase inhibitors should be discontinued if a marked increase in creatinine kinase is observed or myopathy/rhabdomyolysis is diagnosed or suspected.Dose reduction of statins may be needed. Refer to the statin-specific prescribing information for details.
Hydrochlorothiazide: In a pharmacokinetic interaction study, coadministration of multiple-dose hydrochlorothiazide to healthy volunteers receiving fluconazole increased plasma concentrations of fluconazole by 40%. An effect of this magnitude should not necessitate a change in the fluconazole dose regimen in subjects receiving concomitant diuretics.
Ibrutinib: Moderate inhibitors of CYP3A4 such as fluconazole may increase plasma ibrutinib concentrations and increase risk of adverse reactions associated with ibrutinib. If ibrutinib and fluconazole are concomitantly administered, reduce the dose of ibrutinib as instructed in ibrutinib prescribing information and the patient should be frequently monitored for any adverse reactions associated with ibrutinib.
Ivacaftor and fixed dose ivacaftor combinations (e.g., tezacaftor/ivacaftor and ivacaftor/tezacaftor/elexacaftor): Coadministration with ivacaftor, a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator, increased ivacaftor exposure by 3‑fold. If used concomitantly with a moderate inhibitor of CYP3A4, such as fluconazole, a reduction in the dose of ivacaftor (or ivacaftor combination) is recommended as instructed in the ivacaftor (or ivacaftor combination) prescribing information.
Lemborexant: Concomitant administration of fluconazole increased lemborexant Cmax and AUCby approximately 1.6- and 4.2-fold, respectively which is expected to increase risk of adverse reactions, such as somnolence. Avoid concomitant use of fluconazole tablets with lemborexant.
Losartan: Fluconazole inhibits the metabolism of losartan to its active metabolite (E-31 74) which is responsible for most of the angiotensin Il-receptor antagonism which occurs during treatment with losartan. Patients should have their blood pressure monitored continuously.
Lurasidone: Concomitant use of moderate inhibitors of CYP3A4 such as fluconazole may increase lurasidone plasma concentrations. If concomitant use cannot be avoided, reduce the dose of lurasidone as instructed in the lurasidone prescribing information.
Methadone: Fluconazole may enhance the serum concentration of methadone. Dosage adjustment of methadone may be necessary.
Non-steroidal anti-inflammatory drugs: The Cmax and AUC of flurbiprofen were increased by 23% and 81%, respectively, when coadministered with fluconazole compared to administration of flurbiprofen alone. Similarly, the Cmax and AUC of the pharmacologically active isomer [S-(+)-ibuprofen] were increased by 15% and 82%, respectively, when fluconazole was coadministered with racemic ibuprofen (400 mg) compared to administration of racemic ibuprofen alone.
Although not specifically studied, fluconazole has the potential to increase the systemic exposure of other non-steroidal anti-inflammatory drugs (NSAIDs) that are metabolized by CYP2C9 (e.g., naproxen, lornoxicam, meloxicam, diclofenac). Frequent monitoring for adverse events and toxicity related to NSAIDs is recommended. Adjustment of dosage of NSAIDs may be needed.
Olaparib: Moderate inhibitors of CYP3A4 such as fluconazole increase olaparib plasma concentrations; concomitant use is not recommended. If the combination cannot be avoided, reduce the dose of olaparib as instructed in the LYNPARZA® (Olaparib) Prescribing Information.
Oral contraceptives: Two pharmacokinetic studies with a combined oral contraceptive have been performed using multiple doses of fluconazole. There were no relevant effects on hormone level in the 50 mg fluconazole study, while at 200 mg daily, the AUCs of ethinyl estradiol and levonorgestrel were increased 40% and 24%, respectively. Thus, multiple-dose use of fluconazole at these doses is unlikely to have an effect on the efficacy of the combined oral contraceptive.
Oral hypoglycemics: Clinically significant hypoglycemia may be precipitated by the use of fluconazole with oral hypoglycemic agents; one fatality has been reported from hypoglycemia in association with combined fluconazole and glyburide use. Fluconazole reduces the metabolism of tolbutamide, glyburide, and glipizide and increases the plasma concentration of these agents. When fluconazole is used concomitantly with these or other sulfonylurea oral hypoglycemic agents, blood glucose concentrations should be carefully monitored and the dose of the sulfonylurea should be adjusted as necessary. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Phenytoin: Fluconazole increases the plasma concentrations of phenytoin. Careful monitoring of phenytoin concentrations in patients receiving fluconazole and phenytoin is recommended. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Pimozide: Although not studied in vitro or in vivo, concomitant administration of fluconazole with pimozide may result in inhibition of pimozide metabolism. Increased pimozide plasma concentrations can lead to QT prolongation and rare occurrences of torsade de pointes. Coadministration of fluconazole and pimozide is contraindicated.
Prednisone: There was a case report that a liver-transplanted patient treated with prednisone developed acute adrenal cortex insufficiency when a 3 month therapy with fluconazole was discontinued. The discontinuation of fluconazole presumably caused an enhanced CYP3A4 activity which led to increased metabolism of prednisone. Patients on long-term treatment with fluconazole and prednisone should be carefully monitored for adrenal cortex insufficiency when fluconazole is discontinued.
Quinidine: Although not studied in vitro or in vivo, concomitant administration of fluconazole with quinidine may result in inhibition of quinidine metabolism. Use of quinidine has been associated with QT prolongation and rare occurrences of torsade de pointes. Coadministration of fluconazole and quinidine is contraindicated. (See CONTRAINDICATIONS.)
Rifabutin: There have been reports that an interaction exists when fluconazole is administered concomitantly with rifabutin, leading to increased serum levels of rifabutin up to 80%. There have been reports of uveitis in patients to whom fluconazole and rifabutin were coadministered. Patients receiving rifabutin and fluconazole concomitantly should be carefully monitored. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Rifampin: Rifampin enhances the metabolism of concurrently administered fluconazole. Depending on clinical circumstances, consideration should be given to increasing the dose of fluconazole when it is administered with rifampin. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Saquinavir: Fluconazole increases the AUC of saquinavir by approximately 50%, Cmax by approximately 55%, and decreases the clearance of saquinavir by approximately 50% due to inhibition of saquinavir’s hepatic metabolism by CYP3A4 and inhibition of P-glycoprotein. Dosage adjustment of saquinavir may be necessary.
Short-acting benzodiazepines: Following oral administration of midazolam, fluconazole resulted in substantial increases in midazolam concentrations and psychomotor effects. This effect on midazolam appears to be more pronounced following oral administration of fluconazole than with fluconazole administered intravenously. If short-acting benzodiazepines, which are metabolized by the cytochrome P450 system, are concomitantly administered with fluconazole, consideration should be given to decreasing the benzodiazepine dosage, and the patients should be appropriately monitored. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Sirolimus: Fluconazole increases plasma concentrations of sirolimus presumably by inhibiting the metabolism of sirolimus via CYP3A4 and P-glycoprotein. This combination may be used with a dosage adjustment of sirolimus depending on the effect/concentration measurements.
Tacrolimus: Fluconazole may increase the serum concentrations of orally administered tacrolimus up to 5 times due to inhibition of tacrolimus metabolism through CYP3A4 in the intestines. No significant pharmacokinetic changes have been observed when tacrolimus is given intravenously. Increased tacrolimus levels have been associated with nephrotoxicity. Dosage of orally administered tacrolimus should be decreased depending on tacrolimus concentration. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Theophylline: Fluconazole increases the serum concentrations of theophylline. Careful monitoring of serum theophylline concentrations in patients receiving fluconazole and theophylline is recommended. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Tofacitinib: Systemic exposure to tofacitinib is increased when tofacitinib is coadministered with fluconazole. Reduce the dose of tofacitinib when given concomitantly with fluconazole (i.e., from 5 mg twice daily to 5 mg once daily as instructed in the XELJANZ® [tofacitinib] label). (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Tolvaptan: Plasma exposure to tolvaptan is significantly increased (200% in AUC; 80% in Cmax) when tolvaptan, a CYP3A4 substrate, is co-administered with fluconazole, a moderate CYP3A4 inhibitor. This interaction may result in the risk of a significant increase in adverse reactions associated with tolvaptan, particularly significant diuresis, dehydration and acute renal failure. If tolvaptan and fluconazole are concomitantly administered, the tolvaptan dose should be reduced as instructed in the tolvaptan prescribing information and the patient should be frequently monitored for any adverse reactions associated with tolvaptan.
Triazolam: Fluconazole increases the AUC of triazolam (single dose) by approximately 50%, Cmax by 20% to 32%, and increases t½ by 25% to 50 % due to the inhibition of metabolism of triazolam. Dosage adjustments of triazolam may be necessary.
Vinca alkaloids: Although not studied, fluconazole may increase the plasma levels of the vinca alkaloids (e.g., vincristine and vinblastine) and lead to neurotoxicity, which is possibly due to an inhibitory effect on CYP3A4.
Vitamin A: Based on a case report in one patient receiving combination therapy with all-trans-retinoid acid (an acid form of vitamin A) and fluconazole, central nervous system (CNS) related undesirable effects have developed in the form of pseudotumor cerebri, which disappeared after discontinuation of fluconazole treatment. This combination may be used but the incidence of CNS related undesirable effects should be borne in mind.
Voriconazole: Avoid concomitant administration of voriconazole and fluconazole. Monitoring for adverse events and toxicity related to voriconazole is recommended; especially, if voriconazole is started within 24 h after the last dose of fluconazole. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Zidovudine: Fluconazole increases the Cmax and AUC of zidovudine by 84% and 74%, respectively, due to an approximately 45% decrease in oral zidovudine clearance. The half-life of zidovudine was likewise prolonged by approximately 128% following combination therapy with fluconazole. Patients receiving this combination should be monitored for the development of zidovudine-related adverse reactions. Dosage reduction of zidovudine may be considered.
Physicians should be aware that interaction studies with medications other than those listed in the CLINICAL PHARMACOLOGYsection have not been conducted, but such interactions may occur.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Fluconazole showed no evidence of carcinogenic potential in mice and rats treated orally for 24 months at doses of 2.5 mg/kg/day, 5 mg/kg/day, or 10 mg/kg/day (approximately 2 to 7 times the recommended human dose). Male rats treated with 5 mg/kg/day and 10 mg/kg/day had an increased incidence of hepatocellular adenomas.
Fluconazole, with or without metabolic activation, was negative in tests for mutagenicity in four strains of S. typhimurium, and in the mouse lymphoma L5178Y system. Cytogenetic studies in vivo (murine bone marrow cells, following oral administration of fluconazole) and in vitro (human lymphocytes exposed to fluconazole at 1,000 mcg/mL) showed no evidence of chromosomal mutations.
Fluconazole did not affect the fertility of male or female rats treated orally with daily doses of 5 mg/kg, 10 mg/kg, or 20 mg/kg or with parenteral doses of 5 mg/kg, 25 mg/kg, or 75 mg/kg, although the onset of parturition was slightly delayed at 20 mg/kg PO. In an intravenous perinatal study in rats at 5 mg/kg, 20 mg/kg, and 40 mg/kg, dystocia and prolongation of parturition were observed in a few dams at 20 mg/kg (approximately 5 to 15 times the recommended human dose) and 40 mg/kg, but not at 5 mg/kg. The disturbances in parturition were reflected by a slight increase in the number of still born pups and decrease of neonatal survival at these dose levels. The effects on parturition in rats are consistent with the species specific estrogen-lowering property produced by high doses of fluconazole. Such a hormone change has not been observed in women treated with fluconazole. (See CLINICAL PHARMACOLOGY.)
Pregnancy
Teratogenic Effects
Potential for Fetal Harm: Use in pregnancy should be avoided except in patients with severe or potentially life-threatening fungal infections in whom fluconazole may be used if the anticipated benefit outweighs the possible risk to the fetus. A few published case reports describe a pattern of distinct congenital anomalies in infants exposed in utero to high dose maternal fluconazole (400 to 800 mg/day) during most or all of the first trimester. These reported anomalies are similar to those seen in animal studies. Effective contraceptive measures should be considered in women of child-bearing potential who are being treated with fluconazole 400 to 800 mg/day and should continue throughout the treatment period and for approximately 1 week (5 to 6 half-lives) after the final dose. If fluconazole is used during pregnancy, or if the patient becomes pregnant while taking the drug, the patient should be informed of the potential hazard to the fetus. Spontaneous abortions and congenital abnormalities have been suggested as potential risks associated with 150 mg of fluconazole as a single or repeated dose in the first trimester of pregnancy based on retrospective epidemiological studies. There are no adequate and well-controlled studies of fluconazole in pregnant women. (SeeWARNINGS: Potential for Fetal Harm)
Human Data
Case reports describe a distinctive and rare pattern of birth defects among infants whose mothers received high-dose (400 to 800 mg/day) fluconazole during most or all of the first trimester of pregnancy. The features seen in these infants include brachycephaly, abnormal facies, abnormal calvarial development, cleft palate, femoral bowing, thin ribs and long bones, arthrogryposis, and congenital heart disease. These effects are similar to those seen in animal studies.
Epidemiological studies suggest a potential risk of spontaneous abortion and congenital abnormalities in infants whose mothers were treated with 150 mg of fluconazole as a single or repeated dose in the first trimester, but these epidemiological studies have limitations and these findings have not been confirmed in controlled clinical trials.
Animal Data
Fluconazole was administered orally to pregnant rabbits during organogenesis in two studies at doses of 5 mg/kg, 10 mg/kg, and 20 mg/kg and at 5 mg/kg, 25 mg/kg, and 75 mg/kg, respectively. Maternal weight gain was impaired at all dose levels (approximately 0.25 to 4 times the 400 mg clinical dose based on body surface area [BSA] comparison), and abortions occurred at 75 mg/kg (approximately 4 times the 400 mg clinical dose based on BSA); no adverse fetal effects were observed.
In several studies in which pregnant rats received fluconazole orally during organogenesis, maternal weight gain was impaired and placental weights were increased at 25 mg/kg. There were no fetal effects at 5 mg/kg or 10 mg/kg; increases in fetal anatomical variants (supernumerary ribs, renal pelvis dilation) and delays in ossification were observed at 25 mg/kg and 50 mg/kg and higher doses. At doses ranging from 80 to 320 mg/kg (approximately 2 to 8 times the 400 mg clinical dose based on BSA), embryolethality in rats was increased and fetal abnormalities included wavy ribs, cleft palate, and abnormal craniofacial ossification. These effects are consistent with the inhibition of estrogen synthesis in rats and may be a result of known effects of lowered estrogen on pregnancy, organogenesis, and parturition.
Nursing Mothers
Fluconazole was present in low levels in breast milk following administration of a single 150 mg dose, based on data from a study in 10 breastfeeding women who temporarily or permanently discontinued breastfeeding 5 days to 19 months postpartum. The estimated daily infant dose of fluconazole from breast milk (assuming mean milk consumption of 150 mL/kg/day) based on the mean peak milk concentration (2.61 mcg/mL [range: 1.57 to 3.65 mcg/mL] at 5.2 hours post-dose) was 0.39 mg/kg/day, which is approximately 13% of the recommended pediatric dose for oropharyngeal candidiasis. (Labeled pediatric dose is 6 mg/kg/day on the first day followed by 3 mg/kg/day; estimated infant dose is 13% of 3 mg/kg/day maintenance dose). There are no data on fluconazole levels in milk after repeated use or after high-dose fluconazole. A published survey of 96 breastfeeding women who were treated with fluconazole 150 mg every other day (average of 7.3 capsules [range 1 to 29 capsules]) for lactation-associated candida of the breasts reported no serious adverse reactions in infants. Caution should be exercised when fluconazole is administered to a nursing woman.
Pediatric Use
Use in Pediatric Patients for the Treatment of Oropharyngeal Candidiasis
An open-label, randomized, controlled trial has shown fluconazole to be effective in the treatment of oropharyngeal candidiasis in pediatric patients 6 months to 13 years of age. (See CLINICAL STUDIES.)
Use in Pediatric Patients for the Treatment of Candida Esophagitis, Systemic Candida Infections, or Cryptococcal Meningitis
The use of fluconazole in pediatric patients with cryptococcal meningitis, Candida esophagitis, or systemic Candida infections is supported by the efficacy shown for these indications in adults and by the results from several small noncomparative pediatric clinical studies. In addition, pharmacokinetic studies in pediatric patients (See CLINICAL PHARMACOLOGY) have established a dose proportionality between pediatric patients and adults. (See DOSAGE AND ADMINISTRATION.)
In a noncomparative study of fluconazole administered to pediatric patients (from birth to less than 17 years) with serious systemic fungal infections, most of which were candidemia, the effectiveness of fluconazole was similar to that reported for the treatment of candidemia in adults. Of 17 subjects with culture-confirmed candidemia, 11 of 14 (79%) with baseline symptoms (3 were asymptomatic) had a clinical cure; 13/15 (87%) of evaluable patients had a mycologic cure at the end of treatment but two of these patients relapsed at 10 and 18 days, respectively, following cessation of therapy.
The efficacy of fluconazole for the suppression of cryptococcal meningitis was successful in 4 of 5 pediatric patients (4 years to 10 years of age) treated in a compassionate-use study of fluconazole for the treatment of life-threatening or serious mycosis.
There are limited clinical data to support the efficacy of fluconazole for the primary treatment of cryptococcal meningitis in pediatric patients.
The safety profile of fluconazole has been studied in 577 pediatric patients from 1 day to 17 years of age who received doses ranging from 1 to 15 mg/kg/day for 1 to 1,616 days. (See ADVERSE REACTIONS.)
Use in Pediatric Patients on Extracorporeal Membrane Oxygenation (ECMO)
A prospective, open-label, single-center study was conducted to determine the PK and safety of fluconazole in pediatric patients (ages: from birth to 17 years of age) on ECMO (see CLINICAL PHARMACOLOGY). A loading dose of 35 mg/kg is recommended in pediatric patients on ECMO due to increased volume of distribution (see DOSAGE AND ADMINISTRATION).
Use in Prophylaxis of Invasive Candida Infections in Pediatric Patients (premature infants weighing less than 750 grams at birth)
Safety and effectiveness of fluconazole for the prophylaxis of invasive candidiasis in pediatric patients (premature infants weighing less than 750 grams at birth) have not been established.
A prospective, randomized, double-blind, placebo-controlled, multicenter trial was conducted in premature infants weighing less than 750 grams at birth to evaluate the efficacy and safety of prophylactic fluconazole 6 mg/kg administered twice weekly for 6 weeks versus placebo (NCT00734539). Efficacy was assessed using the endpoint of death or candidiasis by study day 49. The results are summarized in Table 4.
Table 4: Death or Candidiasis by Day 49 in Premature Infants Receiving Fluconazole Prophylaxis
Fluconazole
(N=188)
n (%)Placebo
(N=173)
n (%)P-value Difference(95% CI) Death or candidiasis* 33 (17.6) 38 (22.0) 0.2954 -4.4(-12.6, 3.8) Components of endpoint** Death Candidiasis Missing
27 (14.4)
6 (3.2)
2 (1.0)
25 (14.5)
16 (9.2)
1 (0.5)* Subjects with missing data are imputed as having candidiasis or died.
**Subjects may be counted more than once as two fluconazole subjects and four placebo subjects diagnosed with candidiasis subsequently died by day 49.
The most common fatal serious adverse reactions in the fluconazole vs placebo arms, respectively, were necrotizing enterocolitis (NEC), 9 (5%) vs 9 (5%); neonatal bacterial sepsis, 6 (3%) vs 7 (4%); and neonatal respiratory failure, 4 (2%) vs 2 (0.6%).
The most common serious adverse reactions (>5%), reported in patients receiving fluconazole prophylaxis are displayed in Table 5.
Table 5. Serious Adverse Reactions* Occurring in >5% of Infants Receiving Fluconazole Prophylaxis
Adverse Reaction Fluconazole
(N=188)
n(%)Placebo
(N=173)
n (%)Necrotizing Enterocolitis (NEC) 27 (14) 28 (16) Intestinal Perforation (includes ileal/small intestinal perforation) 13 (7) 7 (4) Neonatal Respiratory Arrest/Neonatal Respiratory Failure 13 (7) 4 (2) Bacterial Sepsis, Neonatal 10 (5) 12 (7) *All serious adverse reactions were assessed and recorded up through 30 days after the final dose of study drug.
Serious adverse reactions included both fatal and non-fatal outcomes.
CloseGeriatric Use
In non-AIDS patients, side effects possibly related to fluconazole treatment were reported in fewer patients aged 65 and older (9%, n =339) than for younger patients (14%, n=2240). However, there was no consistent difference between the older and younger patients with respect to individual side effects. Of the most frequently reported (>1%) side effects, rash, vomiting and diarrhea occurred in greater proportions of older patients. Similar proportions of older patients (2.4%) and younger patients (1.5%) discontinued fluconazole therapy because of side effects. In post-marketing experience, spontaneous reports of anemia and acute renal failure were more frequent among patients 65 years of age or older than in those between 12 and 65 years of age. Because of the voluntary nature of the reports and the natural increase in the incidence of anemia and renal failure in the elderly, it is however not possible to establish a causal relationship to drug exposure.
Controlled clinical trials of fluconazole did not include sufficient numbers of patients aged 65 and older to evaluate whether they respond differently from younger patients in each indication. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Fluconazole is primarily cleared by renal excretion as unchanged drug. Because elderly patients are more likely to have decreased renal function, care should be taken to adjust dose based on creatinine clearance. It may be useful to monitor renal function. (See CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION.)
-
ADVERSE REACTIONSFluconazole is generally well tolerated. In some patients, particularly those with serious underlying diseases such as AIDS and cancer, changes in renal and hematological function test results ...
Fluconazole is generally well tolerated.
In some patients, particularly those with serious underlying diseases such as AIDS and cancer, changes in renal and hematological function test results and hepatic abnormalities have been observed during treatment with fluconazole and comparative agents, but the clinical significance and relationship to treatment is uncertain.
In Patients Receiving a Single Dose for Vaginal Candidiasis:
During comparative clinical studies conducted in the United States, 448 patients with vaginal candidiasis were treated with fluconazole, 150 mg single dose. The overall incidence of side effects possibly related to fluconazole was 26%. In 422 patients receiving active comparative agents, the incidence was 16%. The most common treatment-related adverse events reported in the patients who received 150 mg single dose fluconazole for vaginitis were headache (13%), nausea (7%), and abdominal pain (6%). Other side effects reported with an incidence equal to or greater than 1% included diarrhea (3%), dyspepsia (1%), dizziness (1%), and taste perversion (1%). Most of the reported side effects were mild to moderate in severity. Rarely, angioedema and anaphylactic reaction have been reported in marketing experience.
In Patients Receiving Multiple Doses for Other Infections:
Sixteen percent of over 4,000 patients treated with fluconazole in clinical trials of 7 days or more experienced adverse events. Treatment was discontinued in 1.5% of patients due to adverse clinical events and in 1.3% of patients due to laboratory test abnormalities.
Clinical adverse events were reported more frequently in HIV infected patients (21%) than in non-HIV infected patients (13%); however, the patterns in HIV infected and non-HIV infected patients were similar. The proportions of patients discontinuing therapy due to clinical adverse events were similar in the two groups (1.5%).
The following treatment-related clinical adverse events occurred at an incidence of 1% or greater in 4,048 patients receiving fluconazole for 7 or more days in clinical trials: nausea 3.7%, headache 1.9%, skin rash 1.8%, vomiting 1.7%, abdominal pain 1.7%, and diarrhea 1.5%.
Hepato-biliary: In combined clinical trials and marketing experience, there have been rare cases of serious hepatic reactions during treatment with fluconazole. (See WARNINGS.) The spectrum of these hepatic reactions has ranged from mild transient elevations in transaminases to clinical hepatitis, cholestasis and fulminant hepatic failure, including fatalities. Instances of fatal hepatic reactions were noted to occur primarily in patients with serious underlying medical conditions (predominantly AIDS or malignancy) and often while taking multiple concomitant medications. Transient hepatic reactions, including hepatitis and jaundice, have occurred among patients with no other identifiable risk factors. In each of these cases, liver function returned to baseline on discontinuation of fluconazole.
In two comparative trials evaluating the efficacy of fluconazole for the suppression of relapse of cryptococcal meningitis, a statistically significant increase was observed in median AST (SGOT) levels from a baseline value of 30 IU/L to 41 IU/L in one trial and 34 IU/L to 66 IU/L in the other. The overall rate of serum transaminase elevations of more than 8 times the upper limit of normal was approximately 1% in fluconazole-treated patients in clinical trials. These elevations occurred in patients with severe underlying disease, predominantly AIDS or malignancies, most of whom were receiving multiple concomitant medications, including many known to be hepatotoxic. The incidence of abnormally elevated serum transaminases was greater in patients taking fluconazole concomitantly with one or more of the following medications: rifampin, phenytoin, isoniazid, valproic acid, or oral sulfonylurea hypoglycemic agents.
Post-Marketing Experience:
In addition, the following adverse events have occurred during post-marketing experience.
Immunologic: In rare cases, anaphylaxis (including angioedema, face edema and pruritus) has been reported.
Body as a Whole: Asthenia, fatigue, fever, malaise. Cardiovascular: QT prolongation, torsade de pointes. (See PRECAUTIONS.)
Central Nervous System: Seizures, dizziness.
Hematopoietic and Lymphatic: Leukopenia, including neutropenia and agranulocytosis, thrombocytopenia.
Metabolic: Hypercholesterolemia, hypertriglyceridemia, hypokalemia.
Gastrointestinal: Cholestasis, dry mouth, hepatocellular damage, dyspepsia, vomiting.
Other Senses: Taste perversion.
Musculoskeletal System: myalgia.
Nervous System: Insomnia, paresthesia, somnolence, tremor, vertigo.
Skin and Appendages: Acute generalized exanthematous pustulosis, drug eruption including fixed drug eruption, increased sweating, exfoliative skin disorders including Stevens-Johnson syndrome and toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS) (See WARNINGS), alopecia.
CloseAdverse Reactions in Pediatric Patients:
The pattern and incidence of adverse events and laboratory abnormalities recorded during pediatric clinical trials are comparable to those seen in adults.
In Phase II/III clinical trials conducted in the United States and in Europe, 577 pediatric patients, ages 1 day to 17 years were treated with fluconazole at doses up to 15 mg/kg/day for up to 1,616 days. Thirteen percent of pediatric patients experienced treatment-related adverse events. The most commonly reported events were vomiting (5%), abdominal pain (3%), nausea (2%), and diarrhea (2%). Treatment was discontinued in 2.3% of patients due to adverse clinical events and in 1.4% of patients due to laboratory test abnormalities. The majority of treatment-related laboratory abnormalities were elevations of transaminases or alkaline phosphatase.
Percentage of Patients With Treatment-Related Side Effects Fluconazole
(N=577)Comparative Agents
(N=451)With any side effect 13.0 9.3 Vomiting 5.4 5.1 Abdominal pain 2.8 1.6 Nausea 2.3 1.6 Diarrhea 2.1 2.2 Clinical Trials Experience in Pediatric Patients
Safety in Prophylaxis of Invasive Candida Infections in Premature infants weighing less than 750 grams at birth
In a Phase 3 clinical trial of pediatric patients (premature infants weighing less than 750 grams at birth), the incidence of intestinal perforation in infants receiving fluconazole prophylaxis was higher compared to infants receiving placebo (see PRECAUTIONS: Pediatric Use).
Safety in Pediatric Patients Receiving ECMO
A cohort of 20 pediatric patients (1 day to 17 years of age) on ECMO received fluconazole in a prospective, open-label, single-center safety and PK ECMO study. The adverse reaction profile of fluconazole in these patients was similar to that of adult and pediatric non-ECMO patients (See PRECAUTIONS: Pediatric Use).
-
OVERDOSAGEThere have been reports of overdose with fluconazole accompanied by hallucination and paranoid behavior. In the event of overdose, symptomatic treatment (with supportive measures and gastric ...
There have been reports of overdose with fluconazole accompanied by hallucination and paranoid behavior.
In the event of overdose, symptomatic treatment (with supportive measures and gastric lavage if clinically indicated) should be instituted.
Fluconazole is largely excreted in urine. A 3-hour hemodialysis session decreases plasma levels by approximately 50%.
In mice and rats receiving very high doses of fluconazole, clinical effects in both species included decreased motility and respiration, ptosis, lacrimation, salivation, urinary incontinence, loss of righting reflex, and cyanosis; death was sometimes preceded by clonic convulsions.
Close -
DOSAGE AND ADMINISTRATIONDosage and Administration in Adults : Single Dose - Vaginal candidiasis: The recommended dosage of fluconazole for vaginal candidiasis is 150 mg as a single oral dose. Multiple Dose ...
Dosage and Administration in Adults :
Single Dose
Vaginal candidiasis: The recommended dosage of fluconazole for vaginal candidiasis is 150 mg as a single oral dose.
Multiple Dose
SINCE ORAL ABSORPTION IS RAPID AND ALMOST COMPLETE, THE DAILY DOSE OF FLUCONAZOLE IS THE SAME FOR ORAL AND INTRAVENOUS ADMINISTRATION. In general, a loading dose of twice the daily dose is recommended on the first day of therapy to result in plasma concentrations close to steady-state by the second day of therapy.
The daily dose of fluconazole for the treatment of infections other than vaginal candidiasis should be based on the infecting organism and the patient’s response to therapy. Treatment should be continued until clinical parameters or laboratory tests indicate that active fungal infection has subsided. An inadequate period of treatment may lead to recurrence of active infection. Patients with AIDS and cryptococcal meningitis or recurrent oropharyngeal candidiasis usually require maintenance therapy to prevent relapse.
Oropharyngeal candidiasis: The recommended dosage of fluconazole for oropharyngeal candidiasis is 200 mg on the first day, followed by 100 mg once daily. Clinical evidence of oropharyngeal candidiasis generally resolves within several days, but treatment should be continued for at least 2 weeks to decrease the likelihood of relapse.
Esophageal candidiasis: The recommended dosage of fluconazole for esophageal candidiasis is 200 mg on the first day, followed by 100 mg once daily. Doses up to 400 mg/day may be used, based on medical judgment of the patient’s response to therapy. Patients with esophageal candidiasis should be treated for a minimum of three weeks and for at least two weeks following resolution of symptoms.
Systemic Candida infections: For systemic Candida infections including candidemia, disseminated candidiasis, and pneumonia, optimal therapeutic dosage and duration of therapy have not been established. In open, noncomparative studies of small numbers of patients, doses of up to 400 mg daily have been used.
Urinary tract infections and peritonitis: For the treatment of Candida urinary tract infections and peritonitis, daily doses of 50 to 200 mg have been used in open, noncomparative studies of small numbers of patients.
Cryptococcal meningitis: The recommended dosage for treatment of acute cryptococcal meningitis is 400 mg on the first day, followed by 200 mg once daily. A dosage of 400 mg once daily may be used, based on medical judgment of the patient’s response to therapy. The recommended duration of treatment for initial therapy of cryptococcal meningitis is 10 to 12 weeks after the cerebrospinal fluid becomes culture negative. The recommended dosage of fluconazole for suppression of relapse of cryptococcal meningitis in patients with AIDS is 200 mg once daily.
Prophylaxis in patients undergoing bone marrow transplantation: The recommended fluconazole daily dosage for the prevention of candidiasis in patients undergoing bone marrow transplantation is 400 mg, once daily. Patients who are anticipated to have severe granulocytopenia (less than 500 neutrophils cells/mm3) should start fluconazole prophylaxis several days before the anticipated onset of neutropenia, and continue for 7 days after the neutrophil count rises above 1,000 cells/mm3.
Dosage and Administration in Pediatric Patients
Oropharyngeal candidiasis: The recommended dosage of fluconazole for oropharyngeal candidiasis in pediatric patients 6 months and older is 6 mg/kg on the first day, followed by 3 mg/kg once daily. Treatment should be administered for at least 2 weeks to decrease the likelihood of relapse.
Esophageal candidiasis: For the treatment of esophageal candidiasis, the recommended dosage of fluconazole in pediatric patients 6 months and older is 6 mg/kg on the first day, followed by 3 mg/kg once daily. Doses up to 12 mg/kg/day may be used, based on medical judgment of the patient’s response to therapy. Patients with esophageal candidiasis should be treated for a minimum of three weeks and for at least 2 weeks following the resolution of symptoms.
Systemic Candida infections: The following dosing regimens in Table 6 are recommended for pediatric patients to achieve systemic exposures similar to adults for the treatment of systemic Candida infections, i.e., to maintain an AUC0-24 between 400 to 800 mg*h/L.
Table 6: Recommended Dosing Regimens for the Treatment of Systemic Candida Infections in Pediatric Patients
Patient age Dosing regimen 3 months and older A loading dose of 25 mg/kg on the first day (not to exceed 800 mg) followed by 12 mg/kg once daily (not to exceed 400 mg). Birth to 3 months postnatal age and gestational age 30 weeks and above 25 mg/kg on the first day, followed by 12 mg/kg once daily Birth to 3 months postnatal age and gestational age less than 30 weeks 25 mg/kg on the first day, followed by 9 mg/kg once daily Patients with systemic candidiasis should be treated for a minimum of 3 weeks and for at least 2 weeks following the resolution of symptoms.
Dosing in Pediatric Patients on ECMO
The recommended dosage of fluconazole in pediatric patients 3 months and older on ECMO is 35 mg/kg on the first day (not to exceed 800 mg) followed by 12 mg/kg once daily (not to exceed 400 mg).
For patients from birth to 3 months postnatal age, and gestational age less than 30 weeks, a loading dose of 35 mg/kg on the first day followed by 9 mg/kg once daily is recommended. For patients from birth to 3 months postnatal age and gestational age 30 weeks and above, a loading dose of 35 mg/kg on the first day followed by 12 mg/kg once daily is recommended.
Cryptococcal meningitis: For the treatment of acute cryptococcal meningitis, the recommended dosage is 12 mg/kg on the first day, followed by 6 mg/kg once daily. A dosage of 12 mg/kg once daily may be used, based on medical judgment of the patient’s response to therapy. The recommended duration of treatment for initial therapy of cryptococcal meningitis is 10 to 12 weeks after the cerebrospinal fluid becomes culture negative. For suppression of relapse of cryptococcal meningitis in pediatric patients with AIDS, the recommended dose of fluconazole is 6 mg/kg once daily.
Dosage In Patients With Impaired Renal Function :
Fluconazole is cleared primarily by renal excretion as unchanged drug. There is no need to adjust single dose therapy for vaginal candidiasis because of impaired renal function. In patients with impaired renal function who will receive multiple doses of fluconazole, an initial loading dose of 50 mg to 400 mg should be given. After the loading dose, the daily dose (according to indication) should be based on the following summary:
Creatinine Clearance (mL/min) Recommended Dose (%) > 50 100 ≤ 50 (no dialysis) 50 Hemodialysis 100% after each hemodialysis Patients on hemodialysis should receive 100% of the recommended dose after each hemodialysis; on non-dialysis days, patients should receive a reduced dose according to their creatinine clearance.
These are suggested dose adjustments based on pharmacokinetics following administration of multiple doses. Further adjustment may be needed depending upon clinical condition.
When serum creatinine is the only measure of renal function available, the following formula (based on sex, weight, and age of the patient) should be used to estimate the creatinine clearance in adults:
Males: Weight (kg) × (140-age)
72 × serum creatinine (mg/100 mL)Females: 0.85 × above value
Although the pharmacokinetics of fluconazole has not been studied in pediatric patients with renal insufficiency, dosage reduction in pediatric patients with renal insufficiency should parallel that recommended for adults. The following formula may be used to estimate creatinine clearance in pediatric patients:
K × linear length or height (cm)
serum creatinine (mg/100 mL)
(Where K=0.55 for pediatric patients older than 1 year and 0.45 for infants.)CloseAdministration
Fluconazole tablets are administered orally. Fluconazole tablets can be taken with or without food.
-
HOW SUPPLIEDFluconazole tablets USP, 50 mg are peach colored, oval, flat, bevelled edged, uncoated tablets debossed ‘R’ on one side and “143’ on other side and are supplied in bottles of 30, 100, 500 and unit ...
Fluconazole tablets USP, 50 mg are peach colored, oval, flat, bevelled edged, uncoated tablets debossed ‘R’ on one side and “143’ on other side and are supplied in bottles of 30, 100, 500 and unit dose package of 100 (10 x 10).
Bottles of 30 NDC 55111-143-30
Bottles of 100 NDC 55111-143-01
Bottles of 500 NDC 55111-143-05
Unit Dose Package of 100 (10 x 10) NDC 55111-143-78
Fluconazole tablets USP, 100 mg are peach colored, oval, flat, bevelled edged, uncoated tablets debossed ‘R’ on one side and “144’ on other side and are supplied in bottles of 30, 100, 500 and unit dose package of 100 (10 x 10).
Bottles of 30 NDC 55111-144-30
Bottles of 100 NDC 55111-144-01
Bottles of 500 NDC 55111-144-05
Unit Dose Package of 100 (10 x 10) NDC 55111-144-78
Fluconazole tablets USP, 150 mg are peach colored, oval, flat, bevelled edged, uncoated tablets debossed ‘R’ on one side and “145’ on other side and are supplied in unit dose package of 12 (12 x 1).
Unit Dose Package of 12 (12 x 1) NDC 55111-145-12
Fluconazole tablets USP, 200 mg are peach colored, oval, flat, bevelled edged, uncoated tablets debossed ‘R’ on one side and “146’ on other side and are supplied in bottles of 30, 100, 500 and unit dose package of 100 (10 x 10).
Bottles of 30 NDC 55111-146-30
Bottles of 100 NDC 55111-146-01
Bottles of 500 NDC 55111-146-05
Unit Dose Package of 100 (10 x 10) NDC 55111-146-78
Storage: Store at 20 to 25°C (68 to 77°F) [See USP Controlled Room Temperature].
Maalox® is a registered trademark of Novartis Consumer Health, Inc.
XELJANZ® is a registered trademark of Pfizer Inc.
Rx Only
Manufactured by:
Dr. Reddy’s Laboratories Limited
Bachupally – 500 090 INDIA
Revised: 03/2024
Close -
PATIENT PACKAGE INSERTPATIENT INFORMATION - Fluconazole Tablets USP, 150 mg (floo kon' a zole) This leaflet contains important information about fluconazole tablets. It is not meant to take the place of your ...
PATIENT INFORMATION
Fluconazole Tablets USP, 150 mg (floo kon' a zole)This leaflet contains important information about fluconazole tablets. It is not meant to take the place of your healthcare provider’s instructions. Read this information carefully before you take fluconazole tablets. Ask your healthcare provider if you do not understand any of this information or if you want to know more about fluconazole tablets. What is Fluconazole?
Fluconazole is a prescription medicine used to treat vaginal yeast infections caused by a yeast called Candida. Fluconazole tablets helps stop too much yeast from growing in the vagina so the yeast infection goes away.
Fluconazole tablets are different from other treatments for vaginal yeast infections because it is a tablet taken by mouth. Fluconazole tablets are also used for other conditions. However, this leaflet is only about using fluconazole tablets for vaginal yeast infections. For information about using fluconazole tablets for other reasons, ask your healthcare provider. See the section of this leaflet for information about vaginal yeast infections.What is a vaginal yeast infection?
It is normal for a certain amount of yeast to be found in the vagina. Sometimes too much yeast starts to grow in the vagina and this can cause a yeast infection. Vaginal yeast infections are common. About three out of every four adult women will have at least one vaginal yeast infection during their life.
Some medicines and medical conditions can increase your chance of getting a yeast infection.
If you are pregnant, have diabetes, use birth control pills, or take antibiotics you may get yeast infections more often than other women. Personal hygiene and certain types of clothing may increase your chances of getting a yeast infection. Ask your healthcare provider for tips on what you can do to help prevent vaginal yeast infections.
If you get a vaginal yeast infection, you may have any of the following symptoms:- itching
- a burning feeling when you urinate
- redness
- soreness
- a thick white vaginal discharge that looks like cottage cheese
Do not take fluconazole tablets if you. - take the following medicines:
- quinidine
- erythromycin
- pimozide
- are allergic to fluconazole, the active ingredient in fluconazole tablets, or any of the ingredients in fluconazole tablets. See the end of this Patient Information leaflet for a complete list of ingredients in fluconazole tablets.
Before you take fluconazole tablets, tell your healthcare provider about all of your medical conditions, if you: - have liver problems
- have kidney problems
- have heart problems including heart arrythmias
- have hypokalemia (low potassium)
- are pregnant or plan to become pregnant. Tell your healthcare provider right away if you become pregnant while taking fluconazole tablets. You and your healthcare provider will decide if fluconazole tablets are right for you. If you may become pregnant you should use a birth-control (contraceptive) method while taking fluconazole tablets and for 1 week after your final dose.
- are breastfeeding or plan to breastfeed. Fluconazole can pass into your breastmilk. Talk to your healthcare provider about the best way to feed your baby while you are taking fluconazole tablets.
Especially tell your healthcare provider if you take:- diabetes medicines such as glyburide, tolbutamide, glipizide
- blood pressure medicines like hydrochlorothiazide, losartan, amlodipine, verapamil nifedipine or felodipine
- blood thinners such as warfarin
- cyclosporine, tacrolimus or sirolimus (used to prevent rejection of organ transplants)
- rifampin or rifabutin for tuberculosis
- phenytoin or carbamazepine to control seizures
- theophylline to control asthma
- quinidine (used to correct disturbances in heart rhythm)
- amiodarone (used for treating uneven heartbeats ‘arrhythmias’)
- amitriptyline or nortriptyline for depression
- pimozide for psychiatric illness
- amphotericin B or voriconazole for fungal infections
- erythromycin for bacterial infections
- olaparib, cyclophosphamide or vinca alkaloids such as vincristine or vinblastine for treatment of cancer
- fentanyl, alfentanil or methadone for chronic pain
- ibrutinib used for treating blood cancer
- ivacaftor or ivacaftor combinations, such as tezacaftor/ivacaftor and ivacaftor/tezacaftor/elexacaftor, used to treat cystic fibrosis
- lurasidone used to treat schizophrenia or depression
- lemborexant, used for the treatment of insomnia
- lipid lowering drugs such as atorvastatin, simvastatin, and fluvastatin
- non-steroidal anti-inflammatory drugs including celecoxib, ibuprofen, and naproxen
- prednisone, a steroid used to treat skin, gastrointestinal, hematological or respiratory disorders
- antiviral medications used to treat HIV like saquinavir or zidovudine
- tofacitinib for rheumatoid arthritis
- abrocitinib (used to treat atopic dermatitis, also known as eczema)
- vitamin A nutritional supplement
- tolvaptan used to treat hyponatremia (low levels of sodium in your blood) or to slow kidney function decline
Since there are many brand names for these medicines, check with your healthcare provider or pharmacist if you have any questions.
How should I take fluconazole tablets?
- Take fluconazole tablets exactly as your healthcare provider tells you to.
- Take fluconazole tablets by mouth with or without food.
- If you take too much fluconazole tablets, call your healthcare provider or go to the nearest emergency room right away.
What should I avoid while taking fluconazole tablets?
Fluconazole tablets can cause dizziness and seizures. Do not drive or operate machinery until you know how fluconazole tablets affects you.What are the possible side effects of fluconazole tablets?
Fluconazole tablets may cause serious side effects including:
-
serious liver problems. Some people with serious medical problems have developed serious liver problems that became life-threatening or caused death while taking fluconazole tablets. Sometimes these liver problems can be reversed when you stop taking fluconazole tablets. Tell your healthcare provider right away if you have symptoms of serious liver problems including:
- dark colored urine
- light-colored stools
- vomiting
- severe skin itching
- tiredness
- loss of appetite
- yellowing of the skin and eyes (jaundice)
-
serious allergic reactions: Serious allergic reactions (anaphylaxis) have happened while taking fluconazole tablets. Stop taking fluconazole tablets, call your healthcare provider or go to the nearest hospital emergency room right away if you get any signs or symptoms of an allergic reaction including:
- shortness of breath
- coughing
- wheezing
- fever
- skin rash, hives, blisters or skin peeling
- throbbing of the heart or ears
- swelling of the eyelids
- face, mouth, neck, or any other part of the body
- chills
- serious skin problems. Some people with serious medical problems have developed serious skin problems that have caused death while taking fluconazole tablets. Tell your healthcare provider right away if you develop a rash while taking fluconazole tablets.
- headache
- diarrhea
- nausea or upset stomach
- dizziness
- stomach pain
- changes in the way food tastes
-
adrenal insufficiency: Some people who have taken fluconazole tablets developed adrenal insufficiency that was reversible. Tell your healthcare provider right away if you have symptoms of adrenal insufficiency including:
- long lasting fatigue
- muscle weakness
- loss of appetite
- weight loss
- stomach pain
- dizziness
- nausea
- vomiting
- dizziness or seizures.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store fluconazole tablets?
Store fluconazole tablets at 20 to 25°C (68 to 77°F)
Keep fluconazole tablets and all medicines out of the reach of children.General information about the safe and effective use of fluconazole tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use fluconazole tablets for a condition for which it was not prescribed. Do not give fluconazole tablets to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider for information about fluconazole tablets that is written for health professionals.What are the ingredients in fluconazole tablets?
Active ingredient: fluconazole
Inactive ingredients: dibasic calcium phosphate anhydrous, ferric oxide (iron oxide, red), magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate, and starch.
Manufactured by: Dr. Reddy’s Laboratories Limited, Bachupally – 500 090 INDIA
For Medical Information about fluconazole tablets, please call 1-888-375-3784.This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 03/2024
For Patient Information Sheet, please visit:
www.drreddys.com/pi/fluconazole150mgtabs.pdf
Close -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTIONUnvarnished Area consists of: 2D Barcode, Lot Number, Expiry Date and Serial number. Fluconazole Tablets USP, 50 mg - Container Labeling
Unvarnished Area consists of: 2D Barcode, Lot Number, Expiry Date and Serial number.
Fluconazole Tablets USP, 50 mg - Container Labeling
Close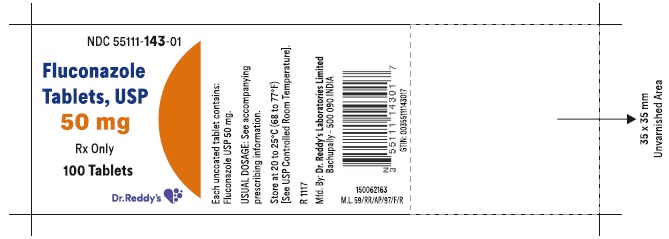
-
PRINCIPAL DISPLAY PANELFluconazole Tablets USP, 100 mg - Container Labeling
-
PRINCIPAL DISPLAY PANELFluconazole Tablets USP, 200 mg - Container Labeling
-
PRINCIPAL DISPLAY PANELFluconazole Tablets USP, 50 mg - Carton Labeling
-
PRINCIPAL DISPLAY PANELFluconazole Tablets USP, 100 mg - Carton Labeling
-
PRINCIPAL DISPLAY PANELFluconazole Tablets USP, 150 mg - Blister Card Labeling
-
PRINCIPAL DISPLAY PANELFluconazole Tablets USP, 150 mg - Carton Labeling
-
PRINCIPAL DISPLAY PANELFluconazole Tablets USP, 200 mg - Carton Labeling
-
INGREDIENTS AND APPEARANCEProduct Information
FLUCONAZOLE fluconazole tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55111-143 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluconazole (UNII: 8VZV102JFY) (Fluconazole - UNII:8VZV102JFY) Fluconazole 50 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FERRIC OXIDE RED (UNII: 1K09F3G675) magnesium stearate (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) povidone (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color PINK (Peach) Score no score Shape OVAL Size 8mm Flavor Imprint Code R;143 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55111-143-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2014 2 NDC:55111-143-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2014 3 NDC:55111-143-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2014 4 NDC:55111-143-78 10 in 1 CARTON 06/20/2014 4 NDC:55111-143-79 10 in 1 DOSE PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076658 06/20/2014 FLUCONAZOLE fluconazole tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55111-144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluconazole (UNII: 8VZV102JFY) (Fluconazole - UNII:8VZV102JFY) Fluconazole 100 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FERRIC OXIDE RED (UNII: 1K09F3G675) magnesium stearate (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) povidone (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color PINK (Peach) Score no score Shape OVAL Size 11mm Flavor Imprint Code R;144 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55111-144-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2014 2 NDC:55111-144-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2014 3 NDC:55111-144-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2014 4 NDC:55111-144-78 10 in 1 CARTON 06/20/2014 4 NDC:55111-144-79 10 in 1 DOSE PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076658 06/20/2014 FLUCONAZOLE fluconazole tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55111-145 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluconazole (UNII: 8VZV102JFY) (Fluconazole - UNII:8VZV102JFY) Fluconazole 150 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FERRIC OXIDE RED (UNII: 1K09F3G675) magnesium stearate (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) povidone (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color PINK (Peach) Score no score Shape OVAL Size 13mm Flavor Imprint Code R;145 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55111-145-12 12 in 1 CARTON 06/20/2014 1 NDC:55111-145-71 1 in 1 DOSE PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076658 06/20/2014 FLUCONAZOLE fluconazole tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55111-146 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluconazole (UNII: 8VZV102JFY) (Fluconazole - UNII:8VZV102JFY) Fluconazole 200 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FERRIC OXIDE RED (UNII: 1K09F3G675) magnesium stearate (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) povidone (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color PINK (Peach) Score no score Shape OVAL Size 14mm Flavor Imprint Code R;146 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55111-146-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2014 2 NDC:55111-146-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2014 3 NDC:55111-146-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2014 4 NDC:55111-146-78 10 in 1 CARTON 06/20/2014 4 NDC:55111-146-79 10 in 1 DOSE PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076658 06/20/2014 Labeler - Dr. Reddy's Laboratories Limited (650562841)
CloseEstablishment Name Address ID/FEI Business Operations Dr. Reddy's Laboratories Limited (FTO III) 918608162 analysis(55111-143, 55111-144, 55111-145, 55111-146) , manufacture(55111-143, 55111-144, 55111-145, 55111-146)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
FLUCONAZOLE tablet
Number of versions: 14
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Sep 27, 2024 | 18 (current) | download |
| May 9, 2024 | 17 | download |
| Sep 6, 2023 | 16 | download |
| Apr 12, 2023 | 15 | download |
| May 25, 2022 | 14 | download |
| Dec 2, 2021 | 13 | download |
| Dec 4, 2020 | 12 | download |
| Mar 19, 2019 | 11 | download |
| Feb 7, 2019 | 10 | download |
| Jan 19, 2018 | 7 | download |
| Sep 23, 2016 | 6 | download |
| Jun 20, 2014 | 4 | download |
| Mar 21, 2014 | 3 | download |
| Jan 5, 2007 | 1 | download |
RxNorm
FLUCONAZOLE tablet
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 197698 | fluconazole 100 MG Oral Tablet | PSN |
| 2 | 197698 | fluconazole 100 MG Oral Tablet | SCD |
| 3 | 197699 | fluconazole 150 MG Oral Tablet | PSN |
| 4 | 197699 | fluconazole 150 MG Oral Tablet | SCD |
| 5 | 197700 | fluconazole 200 MG Oral Tablet | PSN |
| 6 | 197700 | fluconazole 200 MG Oral Tablet | SCD |
| 7 | 197701 | fluconazole 50 MG Oral Tablet | PSN |
| 8 | 197701 | fluconazole 50 MG Oral Tablet | SCD |
Get Label RSS Feed for this Drug
FLUCONAZOLE tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=d7fa1d79-4cd3-4a55-b74b-b31e82e616a3
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
FLUCONAZOLE tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 55111-143-01 |
| 2 | 55111-143-05 |
| 3 | 55111-143-30 |
| 4 | 55111-143-78 |
| 5 | 55111-143-79 |
| 6 | 55111-144-01 |
| 7 | 55111-144-05 |
| 8 | 55111-144-30 |
| 9 | 55111-144-78 |
| 10 | 55111-144-79 |
| 11 | 55111-145-12 |
| 12 | 55111-145-71 |
| 13 | 55111-146-01 |
| 14 | 55111-146-05 |
| 15 | 55111-146-30 |
| 16 | 55111-146-78 |
| 17 | 55111-146-79 |